On publishing in a journal peer-reviewed by kids, and suggesting it as a first journal new PhD students should be asked to write for
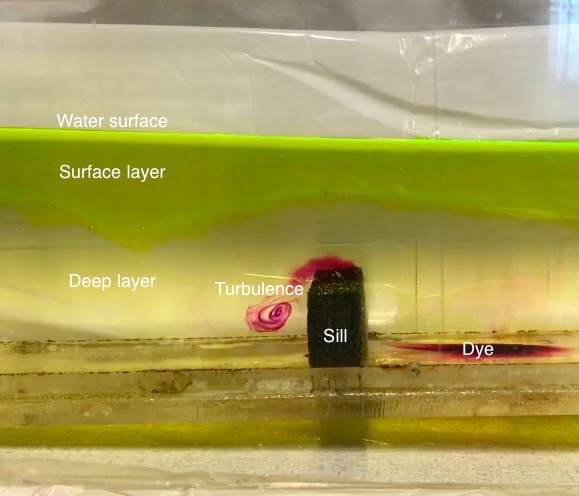
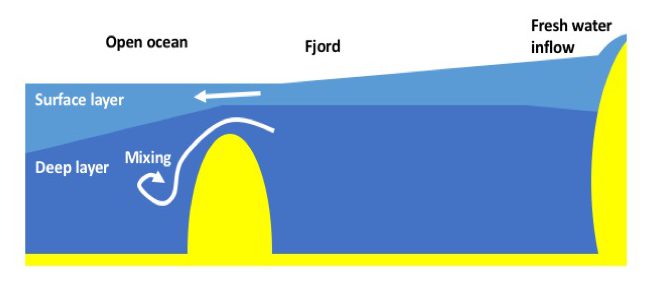
You guys might remember my favourite experiment with the ice cubes melting in freshwater and saltwater. This experiment can be used for almost any teaching purpose (Introduction to experimenting? Check! Thermohaline circulation? Check! Lab safety? Check! Scientific process? Check! And the list goes on and on…) and for any audience (necessary observation skills start a taking the time it takes ice cubes to melt in the easiest case, to observing the finest details of the melt). In short, I love this experiment!
A different format of science communication
After using it in all kinds of settings for years, I wrote up the experiment for Frontiers Young Minds, a journal which is written for, and peer-reviewed by, kids (link to my article). I love the idea of not only tailoring your science communication to the audience of young readers, but making sure that it actually works well for them by including them in the process. Additionally, the peer-reviewers get a great insight into how a publishing process (and thus an important step in science) works, too.
The whole peer-review and publication process was a really positive experience. Speciality chief editor for “Earth and its resources“, Mark Brandon, and the whole team were super responsive and helpful all the way from initial article idea until publication.
Writing for and being peer-reviewed by young readers
Having my writing peer-reviewed by the “young readers” was super interesting. For example, on one of my articles, they commented on how, as kids growing up in the US, they were not familiar with metric units and could I please give them units they could actually relate to? This is an issue I should probably have been aware of, but I totally wasn’t.
Another example from the other article: a different young reader commented that English was their second language, and could I replace difficult words like “puddle” and “dye” with easier words. As a non-native English speaker myself, this feedback was super helpful — I thought that I was writing in an easy language already, but clearly my perception of “easy language” has drifted into specialized vocabulary — super valuable feedback!
And then both teams reviewing both my articles had a science mentor helping them, and also commenting him/herself on the article and how the review process with the kids went and suggesting further edits, that would make it easier for kids to work with the article.

Illustration by Jessie Miller for Frontiers Young Minds, used with permission
And then, of course, there are Jessie Miller‘s super cute illustrations! After seeing what she did for my first article, I couldn’t wait to see what would happen for this one, and I am super excited about another illustration that makes me feel completely understood and seen.
Writing your first ever article for FYM?
So all in all, publishing with FYM is something I would totally recommend to anyone. And I would even go so far as to recommend it as the first article that PhD students should be asked to write. Why?
- Articles for FYM can be written on “core concepts”, which can mean basically writing a literature review on the topic you are about to write a PhD thesis on, and one that is broken down so far that you will really have to have understood things. There is this saying attributed to basically all science educators in one form or another, that only if you can explain your topic to a child, do you actually understand it yourself. So explaining to children is actually a super helpful step in the process of getting into a topic yourself.
- Writing something that is designed to be understood by a wide variety of audiences is really useful for another reason, too: to give to all your family and friends as an easy insight into what it is you are spending all your time on.
- The feedback you get on how you talk about your topic will be helpful for all future communications about it; Practicing scicomm as early as possible is always a good idea :-)
- Having a really positive publishing experience is a great start into a PhD, because surely other kinds of experiences will follow sooner or later. The submission through the uploads and forms and stuff works the same way for FYM as for all other journals (including the “oh crap, they want the images in a different format than I prepared them in! Let’s google how to convert them”, “Really? They need an abstract? Maybe I should have read the instructions more carefully…”, or “They are really counting the words on the submission! So now I need to cut an extra paragraph that I thought I could get away with…” surprises that are typical for the “Let me quickly submit this article and go for lunch! Oh wait, half a day later and I am still nowhere near the end of the process” experience that is so common when submitting articles. At the same time, the stakes feel a little lower for this kind of article, since as an early PhD student, you are writing about other people’s work, not yet your own (at least when writing a core concept article, there is also the “cutting edge research” article type, in which you are writing about some newly published article of yours). And then, as I described above, the whole process is really positive and friendly and supportive throughout, even though all the steps are the same as for any other journal (Waiting for the editor to send the article out to the reviewers. Seeing that stuff is waiting on a desk somewhere and compulsively checking every day whether it has been moved on and the email notification just didn’t make it through. Replying to a reviewer. That kind of things). So I believe that it’s a really good way to be introduced to the publishing process without being pushed into super cold water right away, building up confidence for later submissions of your own work.
- FYM announces new articles on their social media (with lovely tweets!), which have a fairly wide reach, well above what most of us have, and that’s a great opportunity to be seen as authority on a topic by a large number of potentially interested people. Great opportunity to expand your network!
- And, as I said before, I just love the illustrations and I would imagine that having something like this when you start working on a new topic would be super exciting and motivating :-)
What do you think? Will you suggest writing a FYM article to all your new PhD students now?
P.S.: Here are the links to my FYM articles again: “How does ice form in the sea?” and “When Water Swims in Water, Will it Float, or Will it Sink? Or: What Drives Currents in the Ocean?“.