
Tag: temperature





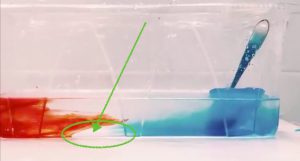
Brine rejection and overturning, but not the way you think! Guest post by Robert Dellinger
It’s #KitchenOceanography season! For example in Prof. Tessa M Hill‘s class at UC Davis. Last week, her student Robert Dellinger posted a video of an overturning circulation on Twitter that got me…
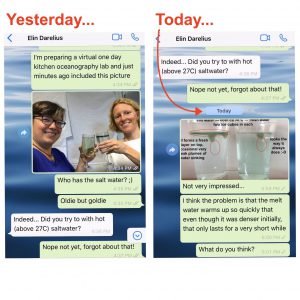
On melting ice cubes and molecular diffusion of heat
First of all, let me say how much I love having chats like the one Elin and I had over the weekend (which you only see the very beginning of…

Temperature dependency of molecular diffusion, and convection taking over
I saw the idea for this experiment on Instagram (Max is presenting it for @glaeserneslabor) and had to try it, too! The idea is to put drops of dye into…
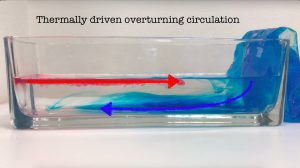
Thermally driven overturning circulation
Today was the second day of tank experiments in Torge’s and my “dry theory 2 juicy reality” teaching innovation project. While that project is mainly about bringing rotating tanks into…

Demonstration: Nansen’s “dead water” in a tank!
A ship that is continuously pulled with a constant force suddenly slows down, stops, and then continues sailing as if nothing ever happened? What’s going on there? We will investigate…

Accidental double-diffusive mixing
When setting up the stratification for the Nansen “dead water” demo (that we’ll show later today, and until then I am not allowed to share any videos, sorry!), I went…

Experiment: Temperature-driven circulation
My favorite experiment. Quick and easy and very impressive way to illustrate the influence of temperature on water densities. This experiment is great if you want to talk about temperature…

Melting ice cubes experiment — observing the finer details
If you don’t know my favourite experiment for practically all purposes yet (Introduction to experimenting? Check! Thermohaline circulation? Check! Lab safety? Check! Scientific process? Check! And the list goes on…
Using the “melting ice cube” experiment to let future instructors experience inquiry-based learning.
Using the “melting ice cube” experiment to let future instructors experience inquiry-based learning. I recently (well, last year, but you know…) got the chance to fill in for a colleague…

One of the most exciting things about work travel?
One of the most exciting things about work travel? Staying in tons of different hotels, which all have different opportunities to play with water. For example at a recent team…

The importance of playing in outreach activities.
Some time ago, I wrote two blog posts on the importance of playing in outreach activities for the EGU’s blog’s “educational corner” GeoEd. Both have now been published, check them…

My favorite demonstration of the coolest mixing process: Salt fingering!
I am updating many of my old posts on experiments and combining multiple posts on the same topic to come up with a state-of-the-art post, so you can always find the…

Temperature-driven overturning experiment – the easy way
In my last post, I showed you the legendary overturning experiment. And guess what occurred to me? That there is an even easier way to show the same thing. No gel…

A very simple overturning experiment for outreach and teaching
For one of my side-projects I needed higher-resolution photos of the overturning experiment, so I had to redo it. Figured I’d share them with you, too. You know the experiment: gel…

Ice cubes melting at the bottom of the beakers
Because surely there is one more post in this topic? ;-) For those of you who haven’t heard about the “melting ice cube” obsession of mine, please check out the…

Why folic acid might be good for people, but not so good for tank experiments
I had to do the complete series of experiments, of course… The other day I mentioned that I had used salt from my kitchen for the “ice cubes melting in…

Melting ice cubes, again
Somehow I am stuck on this demonstration! I can’t let go of this experiment. Last time I posted about it, someone (Hallo Papa!) complained about the background and how I…

Conducting experiments at EMSEA14
Kristin’s and my workshop at EMSEA14. As I mentioned before, Kristin Richter and I are running the workshop “Conducting oceanographic experiments in a conventional classroom anywhere” at the European Marine Science…

The icy elevator
Weird things happening when ice cubes melt. Remember I said that there were weird and wonderful things going on when I last ran the melting ice cubes in salt and…

Melting ice cubes reloaded
Or why you should pay attention to the kind of salt you use for your experiments. The melting ice cubes in salt and fresh water is one of my favorites that…

Salt fingering
My absolute favorite experiment ever: salt fingering. I know I’ve said it before about another experiment, even today, but this is my absolute favorite experiment and I still get endlessly…

Thermally driven circulation
One of my all-time favorite experiments. The salt group got a bit bored from watching ice cubes melt, so I suggested they look at temperature differences for a change, and…

Effects of temperature and salinity on density and stratification
Removing a barrier between waters of different densities and watching what happens. (deutscher Text unten) Today, one of the groups performed a classical experiment (shown for example here) – but…

Lava
Don’t you just love lava lamps? I got a lot of weird looks when I excitedly told people about two years ago that I had just bought a lava lamp.…

Tea and milk
More physics applications connected to tea. After the frustrations of taking pictures of steam in my last post, I decided that I could use the very same cute mug to…

Modeling the Denmark Strait Overflow
Ha, this is a bad pun. We are modeling the Denmark Strait Overflow – but in a non-numerical, small-scale-and-playdough kind of way. More than a year ago, Kjetil and I ran…

Help! Equation of State?
Is there an equation of state for hypersaline water at very cold temperatures? A friend of mine is looking to calculate changes in density of a hypersaline Antarctic lake from summer…

Molecular diffusion of heat and salt
Why heat and salt diffuse at different rates. Why do heat and salt diffuse at different rates? This seems to always be puzzling students when talking about double diffusion. Well,…

Salt fingering – DIY
How to easily set up the stratification for the salt fingering process. Setting up stratifications in tanks is a pain. Of course there are sophisticated methods, but when you want…

Diffusive layering. Or: This is not a trick question!
The “other” double-diffusive mixing process. After having talked extensively about double diffusive mixing in my courses, I tend to assume that students not only remember that there is such thing as…

Salt fingering
How to show my favorite oceanographic process in class, and why. As I mentioned in this post, I have used double-diffusive mixing extensively in my teaching. For several reasons: Firstly,…

Double-diffusive mixing
On the coolest process in oceanography. My favorite oceanographic process, as all of my students and many of my acquaintances know, is double-diffusive mixing. Look at how awesome it is:…

How sound is refracted towards the regions of minimum speed.
Students acting out the process of sound being refracted towards the region of minimum speed. We’ve been talking about refraction lately. Waves get bent in the direction of lower velocity.…

Measuring temperature.
Students build thermometers. As described in this post, I like to have students build “instruments” to measure the most oceanographic properties (temperature, salinity and density). I find that they appreciate oceanographic…

How a CTD works
Movie on how the most important instrument in oceanography works. On our cruise on the WHOI research vessel Knorr in 2011, Sindre Skrede (find him on twitter or vimeo for many more…

Ice cubes melting in fresh water and salt water (post 2/4)
The “ice cubes melting in fresh water and salt water” experiment the way I usually use it in class. — Edit — For an updated description of this experiment please…

Ice cubes melting in salt water and freshwater (post 1/4)
Experiment to visualize the effects of density differences on ocean circulation. This is the first post in a series on one of my favorite in-class experiments; I have so much…

And even more on density
My favorite experiment. Quick and easy and very impressive way to illustrate the influence of temperature on water densities. Today in the “introduction to oceanography” (GEOF130) we conducted my favorite…

How to measure temperature, salinity and density
Three in-class experiments run in parallel. Great if you want to discuss how properties are measured and what kind of difficulties you might encounter. Temperature, salinity and density are the…