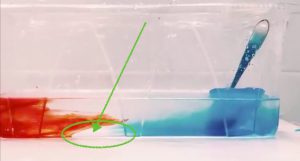
Currently reading To (2025) on “Feedback partnerships: strengthening students’ proactive recipience through co-creating dialogic feedback”
Today I present to you another artefact stemming from my new social media habits (browsing bluesky on the bus to work in the morning, usually finding at least one article…