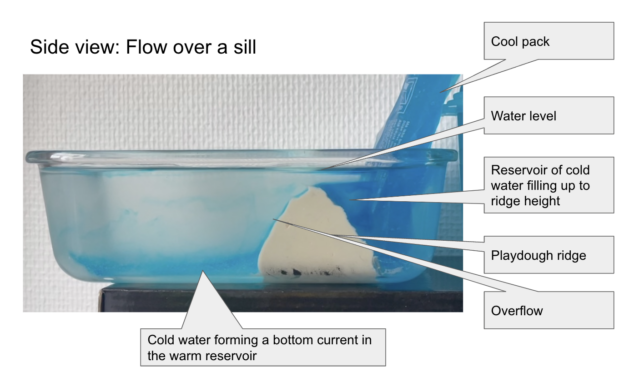
A project near and dear to my heart is using the DIYnamics rotating tank experiments in across-course collaborations. “Older” students, who did experiments the previous year, are trained to then act as guides to “younger” students when they do experiments for the first time, thus lowering the threshold of engaging with equipment, acting as role models when it comes to experimentation, the way to talk about the experiments, and much more. The “younger” students appreciate the interaction, support, and guiding questions, the “older” students realize how much they learned in only a year and what an important role questions play in the learning process.
We started planning this project already before the pandemic, then ran the very first test with 3 paid “older” students in 2020, and then with both full courses, “older” and “younger” students, in 2021 (which is when I took the pictures in this blog post). Then in 2022, we made sure to evaluate the whole thing properly, and that is what, after we presented this project at several conferences already (for example this spring: poster here), is now finally published as
Daae, K., Årvik, A. D., Darelius, E., Glessmer, M. S. (2023). “Student guides: supporting learning from laboratory experiments through across-course collaboration”. Nordic Journal of STEM Education, Vol. 7 No. 1: full papers 2023, p 98-105, DOI: 10.5324/njsteme.v7i1.5093
You can download the pdf here (and you should, it’s a pretty cool project!).