Tag Archives: double-diffusive mixing


Diffusive layering — a super easy experiment!
Day 17 of my 24 days of #KitchenOceanography is about double-diffusive layering, and the post is using “go have a nice latte” as instructions. However, in times of Covid-19 (and a hard lockdown in Germany since Wednesday) that’s unfortunately impossible. And since Lars Henrik said that he was especially curious about this experiment, and today is the day of my “inaugural” lecture back at GFI in Bergen, I thought that was reason enough for a little upgrade to my advent calendar.
So here we go: Looking at diffusive layering in a coffee-and-milk scenario!
The experiment is SUPER easy. The only reasons you might not be aware of this happening are
- You don’t drink coffee in a glas
- You don’t add milk
- You stirr too much and/or too soon
- You drink the coffee too early
So. If you avoid all that, this is what you will see: A stratification with nice layers forming!

All you need to do is
- Make coffee
- Pour coffee into glass
- Drop a teaspoon or two of sugar into the glass (NO STIRRING RIGHT NOW!)
- Pour some milk into the coffee (don’t stress if it looks very turbulent, it’ll settle…)
- Observe layers forming!
- (Optional: When you feel like you’ve seen enough of those layers, stirr CAREFULLY so a little of the sugar gets dissolved into the lowest layers of the coffee-milk-mixture
- Observe a different set of layers forming)
That’s it! Awesome, isn’t it?
Here is a movie the full experiment:
What’s happening here is that cold milk is denser than hot coffee, therefore it sinks to the bottom. But at the interface, there is a fast transfer of heat and a much slower transfer of matter, so the milk gets warmed up and raises until it reaches a level of its own density (the new interface). Within that layer, properties are pretty much homogeneous, but at the interfaces above and below, there are gradients both in temperature and coffee/milk content (salinity in the ocean). So at each interface, a new diffusive layer will form. Over time, many layers develop.
When we stirr in the sugar after some time, we add a new dissolved substance that influences density, and we re-start the diffusive layering process.
Out-takes: Can you guess what happened here? (It does look super awesome, but why is the stratification being eroded?)

I can tell you. The first time I ran the experiment, some sugar stuck to the condensation inside the glass just above water (coffee?) level. As there was more and more water vapor rising from the coffee and more and more condensation collecting on the side of the glass, sometimes some of that sugar sinks down in dense plumes that break through some of the layers (but isn’t it awesome to see how the layers still catch some of the dense plume?!)
Check out the movie of that experiment, it’s awesome!

24 Days of #KitchenOceanography — Double-diffusive mixing with cream and tea

24 Days of #KitchenOceanography — Double-diffusive mixing

Temperature dependency of molecular diffusion, and convection taking over
I saw the idea for this experiment on Instagram (Max is presenting it for @glaeserneslabor) and had to try it, too!
The idea is to put drops of dye into hot and cold water and observe how in hot water the dye is mixed a lot faster than in cold water — after all, molecules in hot water should move a lot more due to more energy and thus more Brownian motion. And we see that nicely in the upper panel of the picture: In hot water, structures look blurred, whereas in the cold water, we nicely see the vortex rings of dye falling into the water.
But what I found super interesting: Molecular diffusion of dye is only the dominant process in the very beginning of the experiment! Very quickly, molecular diffusion of heat is taking over. By warming the dye, we now get a convective flow that moves dye upward in the warm water (see lower panel).
For someone who worked on double-diffusive mixing (i.e. me) this is very exciting: It’s so nice to observe the effects of both diffusion of dye and diffusion of heat in one experiment! And to be able to show how different processes are important at different times.

What’s next? I think next time I’ll use dye at the different temperatures of the two glasses, that should get rid of the convection. Very curious to see what will happen then! :-)

Salt fingers in my overturning experiment
You might have noticed them in yesterday’s thermally driven overturning video: salt fingers!
In the image below you see them developing in the far left: Little red dye plumes moving down into the clear water. But wait, where is the salt? In this case, the “double” in double diffusion comes from heat and dye which are diffusing at different rates. As temperature’s molecular diffusion is about 100x faster than that of salt (or other things that have to physically change their distribution, rather than just bump into each other to transfer energy), the red and clear water quickly have the same temperature, but then the red dye makes the red water more dense, hence it sinks.

Over time, those fingers become more and more clearly visible…

Until after a couple of minutes, we see that they are really contributing to mixing between the two layers.

Even though double diffusive mixing happens in the ocean, too, the scaling of these fingers is of course totally off if we think of this tank as for example the northern half of the Atlantic. But then so is the density stratification… But it’s always good to keep in mind that while this experiment is showing some things quite nicely, there are also things that are artefacts of the way the experiment is set up and that aren’t analogous to how things work in the ocean.
A really nice and very new-to-me way of observing them is from above:

This is a picture that was taken fairly early in the experiment, when the layers hadn’t propagated far yet and the salt fingers weren’t being pulled back by the shear between the layers. But it’s nice to see how the dye is concentrated in those downward moving fingers, isn’t it?

Ostfriesentee — Double diffusion in a tea cup
Showing double-diffusive mixing in tank experiments is a pain if you try to do it the proper way with carefully measured temperatures and salinities. It is, however, super simple, if you go for the quick and dirty route: Cream in tea! Even easier than the “forget the salt, just add food dye” salt fingering experiment I’ve been recommending until now.

The result of double-diffusive mixing of cream in tea is probably familiar to most (see above), but have you ever looked closely at the process?
Below, we pour cold cream into hot tea. The cream initially sinks to the bottom of the tea cup, but then quickly heats up and fingers start raising to the surface of the cup. They are visible as fingers because while the heat has quickly diffused into the cream, the actual mixing of substances takes longer and the opaque milk stays visible in the clear tea. Only when the fingers have risen to the surface the substances begin to mix due to shear and diffusion of substances. Hence the name “double diffusion”: First diffusion of heat, then of particles afterwards.
Pretty cool, isn’t it?
If you happened to stir the tea before pouring the cream, it looks even more awesome. Home-made galaxies :-)
And isn’t it fascinating how the blob of cream in the middle of the cup stays intact for quite some time?
So now you know the only reason why I am drinking black tea: So I can do salt fingering experiments with it! :-)

Accidental double-diffusive mixing
When setting up the stratification for the Nansen “dead water” demo (that we’ll show later today, and until then I am not allowed to share any videos, sorry!), I went into a meeting after filling in layer 4 (the then lowest). When I came back, I wanted to fill in layer 5 as the new bottom layer. For this experiment we want the bottom four layers to have the same density (so we would actually only have one shallow top layer and then a deep layer below [but we can’t make enough salt water at a time for that layer, so I had to split it into four portions]), and I had mixed it as well as I could. But two things happened: a) my salinity was clearly a little fresher than the previous layer, and b) the water in the tank had warmed up and the new water I was adding with layer 5 was cold tap water. So I accidentally set up the stratification for salt fingering: warm and salty over cold and fresh! Can you spot the darker pink fingers reaching down into the slightly lighter pink water? How cool is this??? I am completely flashed. Salt fingering in a 6 meter long tank! :-D


Layered latte: A great real-life example of double-diffusive mixing!
Sometimes sitting in a café for a work meeting with #lieblingskollegin Julia can lead to unexpected discoveries of oceanographic processes — in my latte! It’s those little things that inspire blog posts…
“Kitchen oceanography” brings the ocean to your house or class room!
Oceanography is often taught in a highly theoretical way without much reference to students’ real life experience. Of course a sound theoretical basis is needed to understand the complexity of the climate system, but sometimes a little “kitchen oceanography” — doing experiments on oceanographic topics with household items — goes a long way to raise interest in the kind of processes that are not easily observed in the real world. I’ve previously written a lot about simple experiments you can perform just using plastic cups, water, ice cubes, and a little salt. But sometimes it’s even easier: Sometimes your oceanography is being served to you in a cafe!
Oceanic processes can be observed in your coffee!
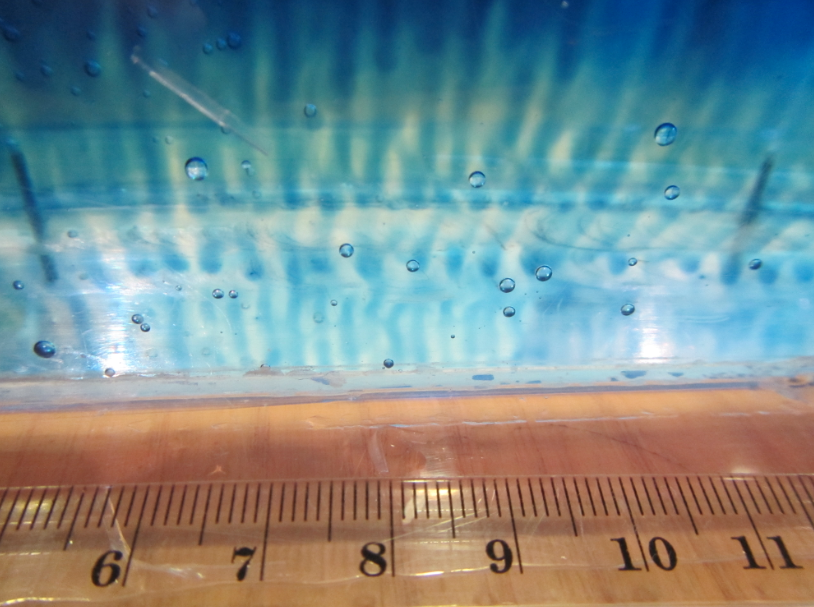
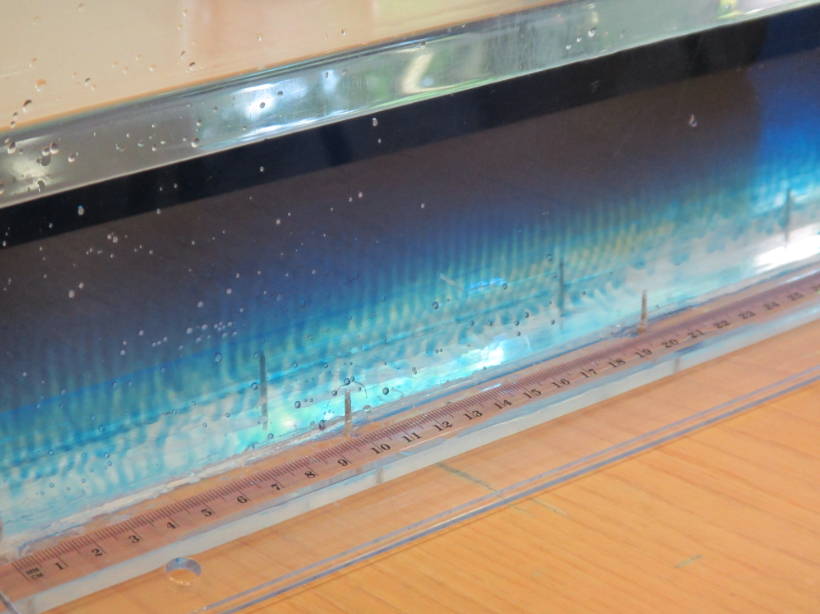
Have you ever looked at your latte and been fascinated by what is going on in there? Many times you don’t just see a homogenous color, but sometimes you see convection cells and sometimes even layers, like in the picture below.
But do you have any ideas why sometimes your latte looks like this and other times it doesn’t?
When you prepare latte in the right way, many layers form
Layers forming in latte (and in the ocean or in engineering applications) are an active research field! In the article “laboratory layered latte” by Xue et al. (2017), the authors describe that the “injection velocity” of espresso into the warm milk has to be above a critical value in order for these pretty structures to form in a latte. They even provide a movie where you can watch the layers develop over a period of several minutes.
The homogeneous layers with sharp boundaries are caused by double-diffusive mixing
Double-diffusive mixing, which is causing the formation of these layers, is the coolest process in oceanography. In a nutshell, double diffusive mixing is caused by two properties influencing density having different rates of molecular diffusion. These different rates can change density in unexpected ways and an initially stable stratification (high density at the bottom, low density on top) can, over time, become statically unstable. And static instability leads to adjustment processes, where water parcels move in order to reach the position in the fluid where they are statically stable — the fluid mixes.
But there are more fascinating things going on with the latte. Would you expect this stratification to remain as clearly visible as it is in the picture above even though the glass is now half empty? I did not! And then check out what happens when you move the glass: Internal waves can travel on the boundaries between layers!
You can use this in class to teach about mixing!
Mixing in the ocean is mostly observed by properties changing over time or in space, and even though (dye) tracer release experiments exist, they are typically happening on scales that provide information on the large-scale effects of mixing and not so much on the mixing itself. And they are difficult to bring inside the classroom! But this is where kitchen oceanography and experiments on double-diffusive mixing come in. If you need inspiration on how to do that, I’ve recently published an article on this (unfortunately only in German), but there are plenty of resources on this blog, too. Or shoot me an email and we’ll talk!
P.S.: Even though the coffee company is displayed prominently in the pictures above, they did not pay for my coffee (or anything else). But if they’d be interested and make me a good offer, I’d definitely write up some fun stuff on learning oceanography with coffee for them ;-)
Experiment: Double-diffusive mixing (salt fingering)
On the coolest process in oceanography.
My favorite oceanographic process, as all of my students and many of my acquaintances know, is double-diffusive mixing. Look at how awesome it is:
Double-diffusive mixing happens because heat and salt’s molecular diffusion are very different: Heat diffuses about a factor 100 faster than salt. This can lead to curious phenomena: Bodies of water with a stable stratification in density will start to mix much more efficiently than one would have thought.
In the specific case of a stable density stratification with warm, salty water over cold, fresh water, finger-like structures form. Those structures are called “salt fingers”, the process is “salt fingering”.

Salt fingering occuring with the red food dye acting as “salt”.
Even though salt fingers are tiny compared to the dimensions of the ocean, they still have a measurable effect on the oceanic stratification in the form of large-scale layers and stair cases, and not only the stratification in temperature and salinity, but also on nutrient availability in the subtropical gyres, for example, or on CO2 drawdown.
Over the next couple of posts, I will focus on double diffusive mixing, but less on the science and more on how it can be used in teaching. (If you want to know more about the science, there are tons of interesting papers around, for example my very first paper)
How to easily set up the stratification for the salt fingering process.
Setting up stratifications in tanks is a pain. Of course there are sophisticated methods, but when you want to just quickly set something up in class (or in your own kitchen) you don’t necessarily want to go through the whole hassle of a proper lab setup.
For double diffusive mixing, there are several methods out there that people routinely use.
For example the hose-and-funnel technique, where the less dense fluid is filled in the tank first and then the denser fluid is slid underneath with the help of a hose and a funnel. And a diffuser at the end of the hose. And careful pouring. And usually a lot more mixing than desired.
Or the plastic-wrap-to-prevent-mixing technique, where the dense fluid is put into the tank, covered by plastic wrap, and then the lighter fluid is poured on top. Then the plastic wrap is removed and by doing so the stratification is being destroyed. (No video because I was frustrated and deleted it right away)
Or some other techniques that I tried and didn’t find too impressive. (No videos either for the same reason as above)
But then accidentally I came across this method (as in: I wanted to show something completely different, but then I saw the salt fingers and was hooked):
Granted, this is not a realistic model of an oceanic stratification. But as you can see towards the end of that movie, that turns out to be a blessing in disguise if you want to talk about the process in detail. As you see in the movie, the salt fingers inside the bottle are much smaller than the salt fingers outside the bottle. Because, clearly, inside the bottle the warm water is cooled both at the interface with the cold water inside the bottle, and by heat conduction through the walls of the bottle, since the water is surrounded by cold water. The warm water that flowed out of the bottle and up towards the water’s surface is only cooled at the interface with the water below (the air above is warmer than the cold water). So this gives you the perfect opportunity to discuss the scaling of salt fingers depending on the stratification without having to go through the pains of actually preparing stratifications with different gradients in temperature or salinity.
In my experience, the best salt fingers happen when you use hot water with dye (as the warm and salty top layer) and cold fresh water below. Salt fingers develop quickly, you don’t have the hassle of hitting the exact temperatures or salinities to make the density stratification statically stable, yet unstable in salinity, and it ALWAYS works.
And look at how beautiful it looks! Do you understand why I LOVE double diffusion?
—
P.S.: This text originally appeared on my website as a page. Due to upcoming restructuring of this website, I am reposting it as a blog post. This is the original version last modified on November 4th, 2015.