Tag Archives: salt fingering


Salt fingers in my overturning experiment
You might have noticed them in yesterday’s thermally driven overturning video: salt fingers!
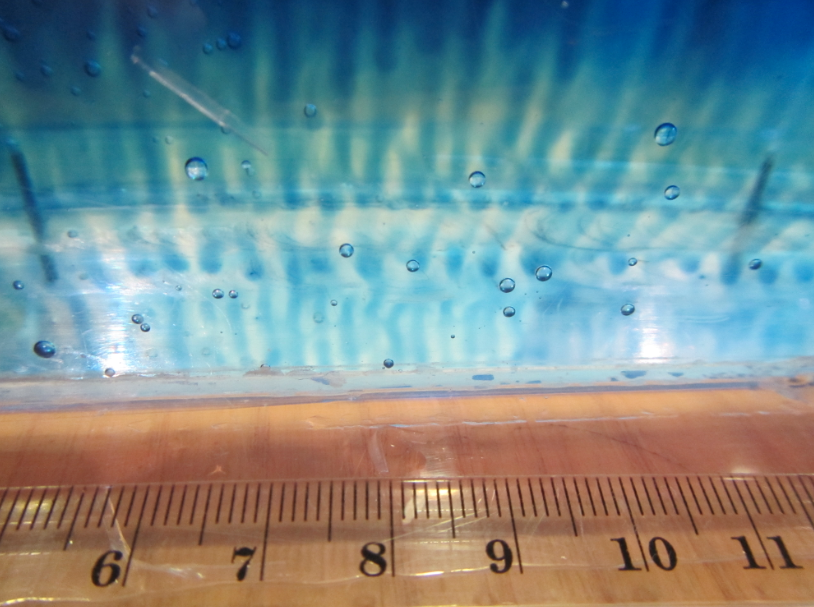
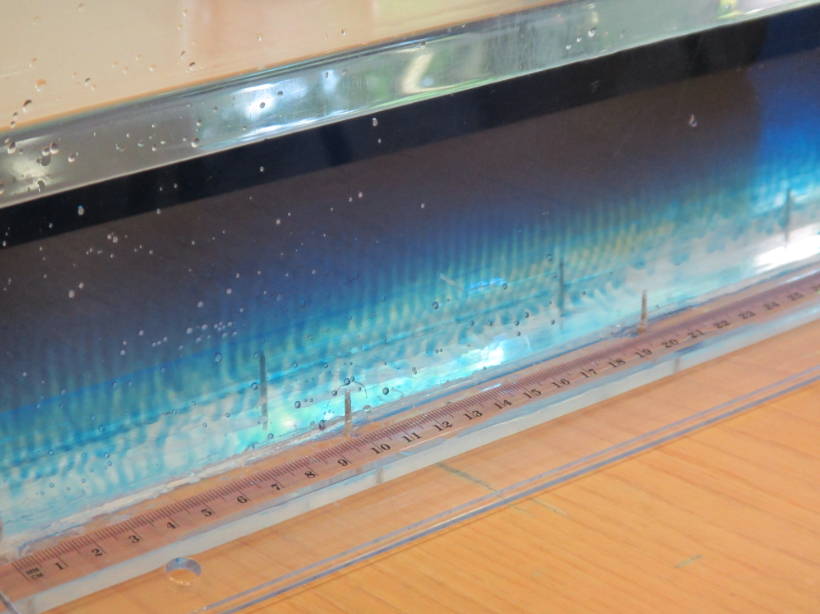
In the image below you see them developing in the far left: Little red dye plumes moving down into the clear water. But wait, where is the salt? In this case, the “double” in double diffusion comes from heat and dye which are diffusing at different rates. As temperature’s molecular diffusion is about 100x faster than that of salt (or other things that have to physically change their distribution, rather than just bump into each other to transfer energy), the red and clear water quickly have the same temperature, but then the red dye makes the red water more dense, hence it sinks.

Over time, those fingers become more and more clearly visible…

Until after a couple of minutes, we see that they are really contributing to mixing between the two layers.

Even though double diffusive mixing happens in the ocean, too, the scaling of these fingers is of course totally off if we think of this tank as for example the northern half of the Atlantic. But then so is the density stratification… But it’s always good to keep in mind that while this experiment is showing some things quite nicely, there are also things that are artefacts of the way the experiment is set up and that aren’t analogous to how things work in the ocean.
A really nice and very new-to-me way of observing them is from above:

This is a picture that was taken fairly early in the experiment, when the layers hadn’t propagated far yet and the salt fingers weren’t being pulled back by the shear between the layers. But it’s nice to see how the dye is concentrated in those downward moving fingers, isn’t it?
Experiment: Double-diffusive mixing (salt fingering)
On the coolest process in oceanography.
My favorite oceanographic process, as all of my students and many of my acquaintances know, is double-diffusive mixing. Look at how awesome it is:
Double-diffusive mixing happens because heat and salt’s molecular diffusion are very different: Heat diffuses about a factor 100 faster than salt. This can lead to curious phenomena: Bodies of water with a stable stratification in density will start to mix much more efficiently than one would have thought.
In the specific case of a stable density stratification with warm, salty water over cold, fresh water, finger-like structures form. Those structures are called “salt fingers”, the process is “salt fingering”.

Salt fingering occuring with the red food dye acting as “salt”.
Even though salt fingers are tiny compared to the dimensions of the ocean, they still have a measurable effect on the oceanic stratification in the form of large-scale layers and stair cases, and not only the stratification in temperature and salinity, but also on nutrient availability in the subtropical gyres, for example, or on CO2 drawdown.
Over the next couple of posts, I will focus on double diffusive mixing, but less on the science and more on how it can be used in teaching. (If you want to know more about the science, there are tons of interesting papers around, for example my very first paper)
How to easily set up the stratification for the salt fingering process.
Setting up stratifications in tanks is a pain. Of course there are sophisticated methods, but when you want to just quickly set something up in class (or in your own kitchen) you don’t necessarily want to go through the whole hassle of a proper lab setup.
For double diffusive mixing, there are several methods out there that people routinely use.
For example the hose-and-funnel technique, where the less dense fluid is filled in the tank first and then the denser fluid is slid underneath with the help of a hose and a funnel. And a diffuser at the end of the hose. And careful pouring. And usually a lot more mixing than desired.
Or the plastic-wrap-to-prevent-mixing technique, where the dense fluid is put into the tank, covered by plastic wrap, and then the lighter fluid is poured on top. Then the plastic wrap is removed and by doing so the stratification is being destroyed. (No video because I was frustrated and deleted it right away)
Or some other techniques that I tried and didn’t find too impressive. (No videos either for the same reason as above)
But then accidentally I came across this method (as in: I wanted to show something completely different, but then I saw the salt fingers and was hooked):
Granted, this is not a realistic model of an oceanic stratification. But as you can see towards the end of that movie, that turns out to be a blessing in disguise if you want to talk about the process in detail. As you see in the movie, the salt fingers inside the bottle are much smaller than the salt fingers outside the bottle. Because, clearly, inside the bottle the warm water is cooled both at the interface with the cold water inside the bottle, and by heat conduction through the walls of the bottle, since the water is surrounded by cold water. The warm water that flowed out of the bottle and up towards the water’s surface is only cooled at the interface with the water below (the air above is warmer than the cold water). So this gives you the perfect opportunity to discuss the scaling of salt fingers depending on the stratification without having to go through the pains of actually preparing stratifications with different gradients in temperature or salinity.
In my experience, the best salt fingers happen when you use hot water with dye (as the warm and salty top layer) and cold fresh water below. Salt fingers develop quickly, you don’t have the hassle of hitting the exact temperatures or salinities to make the density stratification statically stable, yet unstable in salinity, and it ALWAYS works.
And look at how beautiful it looks! Do you understand why I LOVE double diffusion?
—
P.S.: This text originally appeared on my website as a page. Due to upcoming restructuring of this website, I am reposting it as a blog post. This is the original version last modified on November 4th, 2015.
Experiment: Temperature-driven circulation
My favorite experiment. Quick and easy and very impressive way to illustrate the influence of temperature on water densities.
This experiment is great if you want to talk about temperature influencing density. Although it doesn’t actually show anything different from a temperature driven overturning experiment, where circulation is determined by hot water rising and cold water sinking, somehow this experiment is a lot more impressive. Maybe because people are just not used to see bottles pouring out with the water coming out rising rather than plunging down, or maybe because the contrast of the two bottles where one behaves exactly as expected and the other one does not?
Anyway, it is really easy to do. All you need is a big jar and two small bottles. Cold water in one of the small bottles is dyed blue, hot water in the other small bottle is dyed red. Both are inserted in the jar filled with lukewarm water (movie below).
Using bottles with a narrower neck than mouth is helpful if you want to use the opportunity to talk about not only temperature-driven circulation, but also about double-diffusive mixing (which you see in form of salt fingers inside the red bottle in the picture above).
Isn’t this beautiful?
—
P.S.: This text originally appeared on my website as a page. Due to upcoming restructuring of this website, I am reposting it as a blog post. This is the original version last modified on December 2nd, 2015.
My favorite demonstration of the coolest mixing process: Salt fingering!
I am updating many of my old posts on experiments and combining multiple posts on the same topic to come up with a state-of-the-art post, so you can always find the best materials on here. And today I would like to present you my favorite experiment: Salt fingering!
Check out the new page I made for salt fingering!
As you guys might have noticed, I’ve been playing around with my site a quite bit. My blog has moved to mirjamglessmer.com/blog in order to make room for static pages of my favorite experiments or teaching tips right at the landing site mirjamglessmer.com. What do you think? Good idea? Did you notice anything that isn’t quite working yet or do you have advice or wishes? Let me know!
Salt fingering
How to show my favorite oceanographic process in class, and why.
As I mentioned in this post, I have used double-diffusive mixing extensively in my teaching. For several reasons: Firstly, I think that the process is just really cool (watch the movie in this post and tell me that it isn’t!!!) and that the experiments are neat and that everybody will surely be as excited about them as I am. Secondly, because it shows that understanding of small processes can be really important in order to understand the whole eco- and even climate system. And thirdly, because it helps to demonstrate a way of thinking about oceanography.
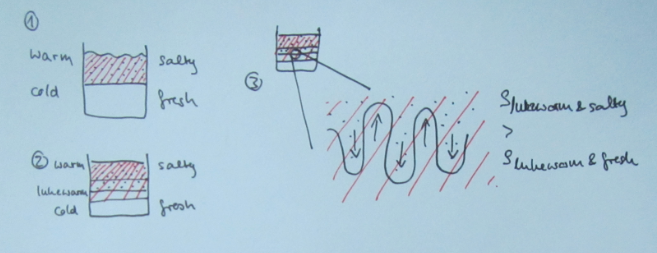
When I introduce salt fingering, I talk students through the process in very small steps. It goes something like this (Numbering is referring to the sketch below):
1) Initially, you have a stratification where warm and salty overlies cold and fresh water. This stratification is stable in density (meaning the influence of the temperature stratification on density outweighs that of the salinity stratification).
2) Since molecular diffusion of temperature is about a factor 100 faster than that of salinity (we will talk about why that is in a later blog post), the interface in salinity is initially basically unchanged, whereas a temperature exchange is happening across that interface, and a layer of medium temperature is forming.
3) At the salinity interface, we now have a stratification that is no longer stable in density: while the water now has the same temperature in a thin layer above and below the interface, it is still more salty on top and less salty below the interface. This means that the saltier water in this thin layer is denser than the less salty water below. This leads to finger-shaped instabilities at the interface: The salty water will sink and the fresh water will rise.
The individual salt fingers now have a much larger surface than the original interface, hence molecular diffusion of salt will happen much more efficiently and eventually the salinity inside and outside of the salt fingers will be the same, hence the growth of the fingers will stop.
At the depth where the salt fingers stopped, a new interface has formed. This new interface can also develop salt fingering, leading to a staircase-like structure in temperature and salinity.
After salt fingering has been introduced, there are usually several other occasions where it, or its effects, can be pointed out, like for example when showing this experiment (see picture below), when talking about the hydrographic properties in the area of the Mediterranean outflow or the Arctic, or when talking about nutrients in subtropical gyres.

This is a zoom in on one of the bottles shown in this experiment: In the warm bottle, the red food dye acts as salt to form salt fingers!
While talking about salt fingering, since I focus so much on the process, I have always been under the illusion that students actually understand the reasoning behind it and that they can reproduce and transfer it. Reproduce they can – transfer not so much. Stay tuned for the next post discussing reasons and possible ways around it.