
Tag: salinity



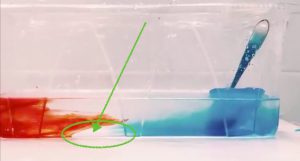
Brine rejection and overturning, but not the way you think! Guest post by Robert Dellinger
It’s #KitchenOceanography season! For example in Prof. Tessa M Hill‘s class at UC Davis. Last week, her student Robert Dellinger posted a video of an overturning circulation on Twitter that got me…
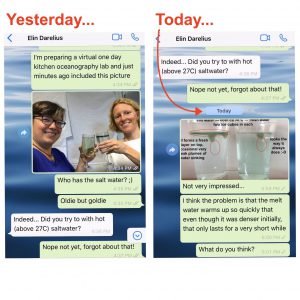
On melting ice cubes and molecular diffusion of heat
First of all, let me say how much I love having chats like the one Elin and I had over the weekend (which you only see the very beginning of…

Temperature dependency of molecular diffusion, and convection taking over
I saw the idea for this experiment on Instagram (Max is presenting it for @glaeserneslabor) and had to try it, too! The idea is to put drops of dye into…

Ice cubes melting in fresh water and salt water
Today we are doing the melting ice cubes experiment in fancy glasses, because Elin is giving a fancy lecture tonight: The Nansen Memorial Lecture of the Norwegian Science Academy in…

A “Siel” – the valve in a dyke that lets freshwater out but no salt water in
Ok now, after complaining about how I dislike mud and the “no water” (i.e. low water) times in the Wadden Sea yesterday, today I’ll tell you about some stuff I…

Demonstration: Nansen’s “dead water” in a tank!
A ship that is continuously pulled with a constant force suddenly slows down, stops, and then continues sailing as if nothing ever happened? What’s going on there? We will investigate…

Accidental double-diffusive mixing
When setting up the stratification for the Nansen “dead water” demo (that we’ll show later today, and until then I am not allowed to share any videos, sorry!), I went…

Guest post: Using seawater to make bread!
Last week I got one of the coolest emails I have ever received: Someone had found my blog while googling for the salt content of seawater in order to use…

Experiment: Temperature-driven circulation
My favorite experiment. Quick and easy and very impressive way to illustrate the influence of temperature on water densities. This experiment is great if you want to talk about temperature…

Melting ice cubes experiment — observing the finer details
If you don’t know my favourite experiment for practically all purposes yet (Introduction to experimenting? Check! Thermohaline circulation? Check! Lab safety? Check! Scientific process? Check! And the list goes on…

Evaporating sea water
How much salt is there in sea water? What concentration do you need before crystals start forming? What will those crystals look like? I am sure those are the kind…

Taking the hydrostatic paradox to the next (water) level
How well do people understand hydrostatics? I am preparing a workshop for tomorrow night and I am getting very bored by the questions that I have been using to introduce…
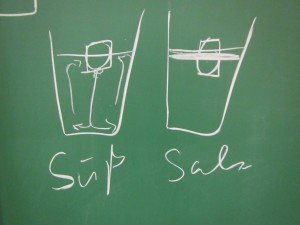
Using the “melting ice cube” experiment to let future instructors experience inquiry-based learning.
Using the “melting ice cube” experiment to let future instructors experience inquiry-based learning. I recently (well, last year, but you know…) got the chance to fill in for a colleague…

When water doesn’t seek its level
Last week we talked about misconceptions related to hydrostatic pressure, and how water always seeks its level. Today I’m gonna show you circumstances in which this is actually not the…

My favorite demonstration of the coolest mixing process: Salt fingering!
I am updating many of my old posts on experiments and combining multiple posts on the same topic to come up with a state-of-the-art post, so you can always find the…

Guest post: Estimating salinity as a homework assignment
Today I am super excited to share a guest post that my awesome friend Joke Lübbecke wrote for us. Joke is a professor in physical oceanography in Kiel, and we…

Ice cubes melting at the bottom of the beakers
Because surely there is one more post in this topic? ;-) For those of you who haven’t heard about the “melting ice cube” obsession of mine, please check out the…

Creating a continuous stratification in a tank, using the double bucket filling method
Because I am getting sick of stratifications not working out the way I planned them. Creating stratifications, especially continuous stratifications, is a pain. Since I wanted a nice stratification for an…

Double overflow
Because sometimes one overflow simply isn’t enough. Finn’s group came up with – and ran – an overflow experiment with many different densities and even more colors. While the movie didn’t…

Forced internal waves in a continuous stratification
Plus all kinds of dyes. (deutscher Text unten) Using the continuous salinity stratification created yesterday, Rolf and Daniel conducted a really cool experiment: They forced internal waves and watched them…

Creating a continuous stratification.
And watching internal waves – a data-model comparison. (deutscher Text unten) In an experiment similar to the one done by the group looking at the effects of temperature and salinity…

Effects of temperature and salinity on density and stratification
Removing a barrier between waters of different densities and watching what happens. (deutscher Text unten) Today, one of the groups performed a classical experiment (shown for example here) – but…

Guest post: The mystery of the cold room
Guest post by Kristin Richter! Today I’m excited to bring to you a guest post from Innsbruck, Austria, written by my friend Kristin Richter. Kristin ran the oceanography lab in Bergen…

Help! Equation of State?
Is there an equation of state for hypersaline water at very cold temperatures? A friend of mine is looking to calculate changes in density of a hypersaline Antarctic lake from summer…

Molecular diffusion of heat and salt
Why heat and salt diffuse at different rates. Why do heat and salt diffuse at different rates? This seems to always be puzzling students when talking about double diffusion. Well,…

Salt fingering – DIY
How to easily set up the stratification for the salt fingering process. Setting up stratifications in tanks is a pain. Of course there are sophisticated methods, but when you want…

Diffusive layering. Or: This is not a trick question!
The “other” double-diffusive mixing process. After having talked extensively about double diffusive mixing in my courses, I tend to assume that students not only remember that there is such thing as…

Salt fingering
How to show my favorite oceanographic process in class, and why. As I mentioned in this post, I have used double-diffusive mixing extensively in my teaching. For several reasons: Firstly,…

Double-diffusive mixing
On the coolest process in oceanography. My favorite oceanographic process, as all of my students and many of my acquaintances know, is double-diffusive mixing. Look at how awesome it is:…

How sound is refracted towards the regions of minimum speed.
Students acting out the process of sound being refracted towards the region of minimum speed. We’ve been talking about refraction lately. Waves get bent in the direction of lower velocity.…

Measuring salinity
Students evaporate water to measure the salinity of a water sample. As described in this post, I like to have students build “instruments” to measure the most oceanographic properties (temperature, salinity…

Tasting sea water reloaded
Doing the “tasting sea water” activity again with a different group of students. A very good introduction to the concept of salinity is the “tasting sea water” activity. Last time…

How a CTD works
Movie on how the most important instrument in oceanography works. On our cruise on the WHOI research vessel Knorr in 2011, Sindre Skrede (find him on twitter or vimeo for many more…

On the structure of fresh water and salt water ice
More details on the structure of fresh water and salt water ice. Fresh water and salt water ice have very different structures as I already discussed in this post. In the…

Melting ice cubes – one experiment, many ways (post 3/4)
Different didactical settings in which the “ice cubes melting in fresh and salt water” experiment can be used. In part 1 and 2 of this series, I showed two different…

How much salt is there in sea water?
Visualization of how much salt is actually contained in sea water. When preparing “sea water samples” for class, it is always astonishing to me how much salt I have to…

Properties of sea ice and fresh water ice
Sea ice and fresh water ice have distinctly different properties that can easily be investigated even in big class rooms. In “on how ice freezes from salt water” I talked…

Ice cubes melting in fresh water and salt water (post 2/4)
The “ice cubes melting in fresh water and salt water” experiment the way I usually use it in class. — Edit — For an updated description of this experiment please…

On how ice freezes from salt water
I’ve been wondering how to best show how sea ice freezes for quite a while. Not just that it freezes, but how brine is rejected. By comparing the structure of…

Ice cubes melting in salt water and freshwater (post 1/4)
Experiment to visualize the effects of density differences on ocean circulation. This is the first post in a series on one of my favorite in-class experiments; I have so much…

How to measure temperature, salinity and density
Three in-class experiments run in parallel. Great if you want to discuss how properties are measured and what kind of difficulties you might encounter. Temperature, salinity and density are the…

Tasting sea water
Hands-on activity on sea water salinity In the first lecture of the “introduction to oceanography” GEOF130 course 2013, we investigated water samples from four different regions: The Mediterranean, the tropical…