Tag Archives: melting ice cubes experiment


24 Days of #KitchenOceanography — Melting ice cubes!
On melting ice cubes and molecular diffusion of heat
First of all, let me say how much I love having chats like the one Elin and I had over the weekend (which you only see the very beginning of above). I had gotten into a bit of a rut kitchen oceanography-wise, which, I am happy to report, is over now! Thanks, Elin! :-)

One of my favourite experiments of all times are the ice cubes melting in fresh water and salt water. I’ve written about this experiment many times before, so if you aren’t familiar with it, check out my introductory post or all the posts related to the original experiment, because now we are going to take it one step further.
Ready?
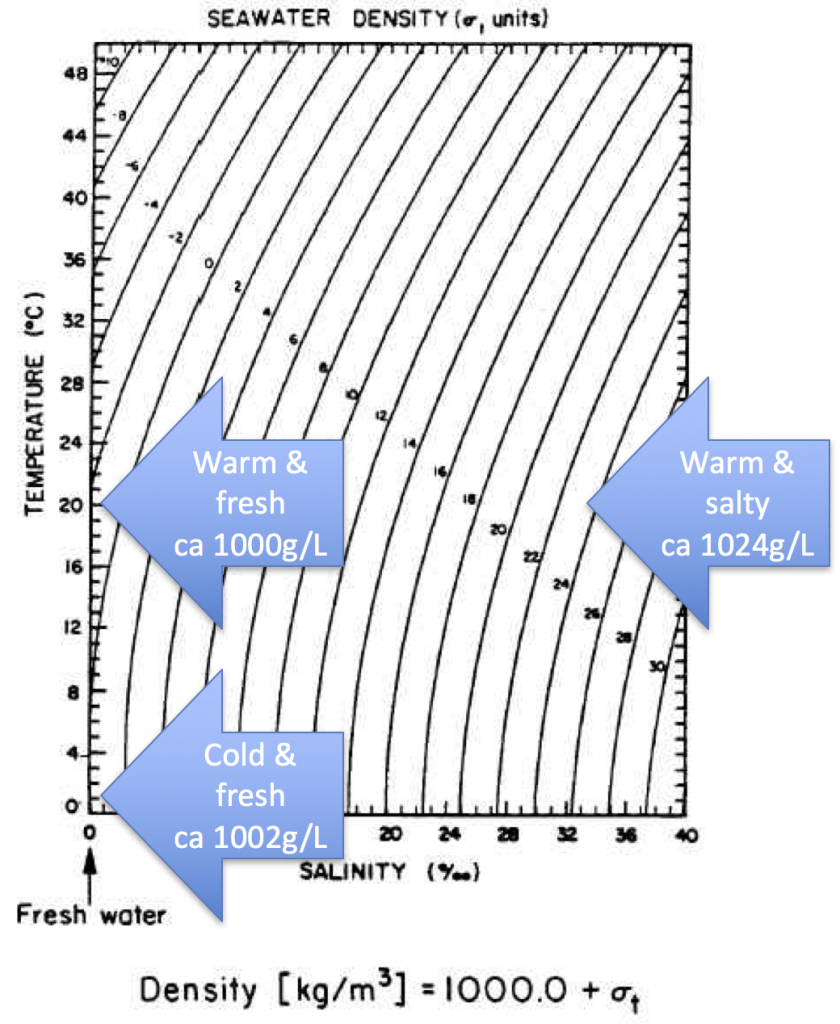
Last year, Elin told me about a conversation she had had with Prof. Emeritus Arne Foldvik (our hero when it comes to tank experiments!) about someone considering towing icebergs from Antarctica to some tropical place to use as freshwater supply. Arne mentioned that when the water in which the iceberg swims gets warmer than 27°C, the situation changes, as in that the iceberg’s melt water now is denser than the warm saltwater it is swimming in. So the assumption from that would be that the melt water would sink, rather than form a layer floating around the iceberg.
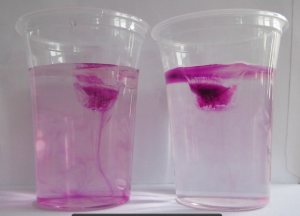
And that’s the experiment I had been wanting to try and only got around to doing when Elin reminded me on Saturday. Results were … a bit disappointing. At least at first:

So what is happening is that even though the melt water is initially denser than the salt water, it doesn’t stay that way for very long, because diffusion of temperature is very fast and the fresh meltwater plumes don’t have to warm up by very much before they are less dense than the warm saltwater, so that happens very quickly.
In the movie below we see evidence of this: Around minute 1 (marked in the video) we can spot plumes of dense water sinking down, but at the same time we very clearly see a (green) freshwater layer forming on top of the salt water.
(The “Happy Birthday Arne” in this movie refers to Arne Foldvik’s 90th Birthday which was yesterday!)
For the experiment, I kinda eyeballed the salinity and also my thermometers might not be the most suitable choice for measuring water temperatures, at least in that temperature range. But as Elin and I discussed as the chat above went on: I think it won’t make a big difference to fiddle with temperatures and salinities, in the end the dominant process will be the molecular diffusion of heat that will always quickly warm the meltwater, making it buoyant. And I actually think that this makes this experiment even more interesting — to show how different processes are acting at the same time, and it’s not always obvious right away which one will be the most important one. Kinda similar to what I showed in yesterday’s post: molecular diffusion of heat will sneak up on you faster than you think :-)
Would the same thing also happen if we didn’t just have small ice cubes with low meltwater “production”, but icebergs, where the meltwater plumes would have a larger volume and so wouldn’t be warmed up as easily? Who knows… But my kitchen is too small to try and I’m too lazy to do the maths, so now it’s your turn! :-)

Ice cubes melting in fresh water and salt water
Today we are doing the melting ice cubes experiment in fancy glasses, because Elin is giving a fancy lecture tonight: The Nansen Memorial Lecture of the Norwegian Science Academy in Oslo! Cheers!
We each had green ice cubes in our glasses, but one of our glasses contained fresh water and the other one salt water, both at room temperature. Can you figure out who got which glass?
This time lapse might give you a clue…
To read more about this experiment, check out this blog post!
Experiment: Ice cubes melting in fresh water and salt water
Explore how melting of ice cubes floating in water is influenced by the salinity of the water. Important oceanographic concepts like density and density driven currents are visualized and can be discussed on the basis of this experiment.
Context
Audience
This hands-on experiment is suited for many different audiences and can be used to achieve a wealth of different learning goals. Audience ranges from first-graders over undergraduates in physical oceanography to outreach activities with the general public. Depending on the audience, this activity can be embedded in very different contexts: For children, either in their physics teaching to motivate learning about concepts like density, or in the context of learning about the climate system and ocean circulation. For college/university students the activity can either be used in physics teaching to get a different view on density; in oceanography/Earth science to talk about ocean circulation and processes that are important there; to motivate the scientific process; or to practice writing lab reports (you can be sure that students will at some point be tasting the water to make sure they didn’t accidentally swap the salt water and fresh water cup – a great teachable moment for a) Never putting anything in your mouth in a laboratory setting, and b) Always documenting exactly what you are doing because stuff that you think you will definitely remember obviously isn’t remembered that clearly after all). For the general public, this is typically a stand-alone activity.
Skills and concepts that students must have mastered
It helps if the concept of density is known, but the experiment can also be used to introduce or deepen the understanding of the concept.
How the activity is situated in the course
I use this activity in different ways: a) as a simple in-class experiment that we use to discuss the scientific method, as well as what needs to be noted in lab journals and what makes a good lab report, or density-driven circulation; b) to engage non-majors or the general public in thinking about ocean circulation, what drives ocean currents, … in one-off presentations.
Goals
Content/concepts goals for this activity
Students learn about concepts that are important not only in physical oceanography, but in any physical or Earth science: density in general; density of water in particular, depending on the water’s temperature and salinity; how differences in density can drive currents both in the model and in the world ocean; how different processes acting at the same time can lead to unexpected results; how to model large scale processes in a simple experiment. After finishing the activity, they can formulate testable hypotheses, are able to reason based on density how a flow field will develop and they can compare the situations in the cups to the “real” ocean.
Higher order thinking skills goals for this activity
Students learn about and practice the use of the scientific method: formulation of hypotheses, testing, evaluating and reformulating.
Other skills goals for this activity
Students practice writing lab reports, making observations, working in groups.
Description and Teaching Materials
Materials
(per group of 2-4 students):
- 1 clear plastic cup filled with room-temperature salt water (35psu or higher, i.e. 7 or more tea spoons of table salt per liter water), marked as salt water (optional)
- 1 clear plastic cup filled with room-temperature fresh water, marked as fresh water (optional)
- 2 ice cubes
- liquid food dye either in drop bottle, with a pipette or with a straw as plunging syphon
Description
Before the experiment is started, students are asked to make a prediction which ice cube will melt faster, the one in salt water or the one in fresh water. Students discuss within their groups and commit to one hypothesis.
Students then place the ice cubes into the cups and start a stop watch/note the time. Students observe one of the ice cube melting faster than the other one. When it becomes obvious that one is indeed melting faster, a drop of food dye can be added on each of the ice cubes to color the melt water. Students take the time until each of the ice cubes has melted completely.
Discussion
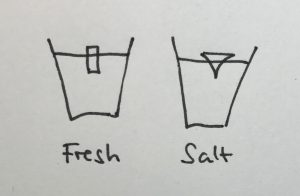
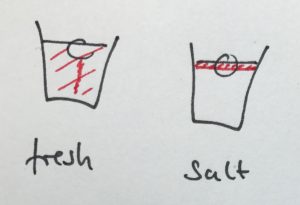
The ice cube in the cup containing the fresh water will melt faster, because the (fresh) melt water is colder than the room-temperature fresh water in the cup. Hence its density is higher and it sinks to the bottom of the cup, being replaced by warmer waters at the ice cube. In contrast, the cold and fresh melt water in the salt water cup is less dense than the salt water, hence it forms a layer on top of the salt water and doesn’t induce a circulation like the one in the fresh water cup. The circulation is clearly visible as soon as the food dye is added: While in the freshwater case the whole water column changes color, only a thin meltwater layer on top of the salt water is colored (for clarification, see images in the presentation below)
Teaching Notes and Tips
Students usually assume that the ice cube in salt water will melt faster than the one in fresh water, “because salt is used to de-ice streets in winter”. Have students explicitly state their hypothesis (“the one in salt water will melt faster!”), so when they measure the time it takes the ice cubes to melt, they realize that their experiment does not support their hypothesis and start discussing why that is the case. (Elicit the misconception, so it can be confronted and resolved!)
My experience with this experiment is that all groups behave very consistently:
- At least 80% of your audience will be very sure that the ice cube in salt water will melt faster than the one in fresh water. The other 20% will give the correct hypothesis, but only because they expect a trick question, and they will most likely not be able to come up with an explanation.
- You can be 100% sure that at least in one group, someone will say “oh wait, which was the salt water again?” which hands you on a plate the opportunity to say “see — this is a great experiment to use when talking about why we need to write good documentations already while we are doing the experiment!”
- You can also be 100% sure that in that group, someone will taste the water to make sure they know which cup contains the salt water. Which lets you say your “see — perfect experiment to talk about lab safety stuff! Never ever put things in your mouth in a lab!”
- You can also be sure, that people come up with new experiments they want to try.
- At EMSEA14, people asked what would happen if the ice cubes were held at the bottom of the beaker.
- At a workshop on inquiry-based learning, people asked what the dye would do if there was no ice in the cups, just salt water and fresh water. Perfect opportunity to say “try! Then you’ll know! And btw — isn’t this experiment perfect to inspire the spirit of research (or however you would say that in English – “Forschergeist” is what I mean!). This is what you see in the pictures in this blogpost.
It is always a good idea to have plenty of spare ice cubes and salt/fresh water at room temperature ready so people can run the experiment again if they decide to either focus on something they didn’t observe well enough the first time round, or try a modified experiment like the ones described above.
A reviewer of this activity asked how easily students overcome the idea that water in the cup has to have just one temperature. In my experience this is not an issue at all – students keep “pointing” and thereby touching the cups, and in the thin-walled plastic cups I typically use the temperature gradient between “cold” melt water and “warm” salt water is easily felt. The (careful!) touching of the cups can also be explicitly encouraged.
Different ways to use this experiment
This experiment can be used in many different ways depending on the audience you are working with.
- Demonstration: If you want to show this experiment rather than having students conduct it themselves, using colored ice cubes is the way to go (see experiment here). The dye focuses the observer’s attention on the melt water and makes it much easier to observe the experiment from a distance, on a screen or via a projector. Dying the ice cubes makes understanding much easier, but it also diminishes the feeling of exploration a lot – there is no mystery involved any more. And remember in order for demonstrations to increase the learning outcome, they need to be embedded in a larger didactical setting, including forming of hypotheses before the experiment is run and debriefing afterwards.
- Structured activity: For an audience with little knowledge about physics, you might want to start with a very structured activity, much like the one described above. Students are handed (non-colored) ice cubes, cups with salt water and fresh water and are asked to make a prediction about which of the ice cubes is going to melt faster. Students test their hypothesis, find the results of the experiment in support with it or not, and we discuss. This is how I usually use this experiment in class (see discussion here).The advantage of using this approach is that students have clear instructions that they can easily follow. Depending on how observant the group is, instructions can be very detailed (“Start the stop watch when you put the ice cubes in the water. Write down the time when the first ice cube has melted completely, and which of the ice cubes it was. Write down the time when the second ice cube has melted completely. …”) or more open (“observe the ice cubes melting”).
- Problem-solving activity: Depending on your goals with this experiment, you could also consider making it a problem-solving activity: You would hand out the materials and ask the students to design an experiment to figure out which of the cups contains fresh water and which salt water (no tasting, of course!). This is a very nice exercise and students learn a lot from designing the experiment themselves.
- Open-ended investigation: In this case, students are handed the materials, knowing which cup contains fresh and salt water. But instead of being asked a specific question, they are told to use the materials to learn as much as they can about salt water, fresh water, temperature and density.As with the problem-solving exercise, this is a very time-intensive undertaking that does not seem feasible in the framework we are operating in. Also it is hard to predict what kind of experiments the students will come up with, and if they will learn what you want them to learn. On the other hand, students typically learn much more because they are free to explore and not bound by a specific instruction from you, so maybe give it a try?
- Problem-based learning: This experiment is also very well suited in a Problem-Based Learning setting, both to work on the experiment itself or, as we did, to have instructors experience how problem-based learning works so they can use it in their own teaching later. Find a suggested case and a description of our experiences with it here.
- Inquiry-based learning: Similarly as with Problem-Based Learning, this experiment can be used to let future instructors experience the method of inquiry-based learning from a student perspective. For my audience, people teaching in STEM, this is a nice case since it is close enough to their topics so they can easily make the transfer from this case to their own teaching, yet obscure enough that they really are learners in the situation.
Pro tip: If you are not quite sure how well your students will be able to cope with this experiment, prepare ice cubes dyed with food coloring and use them in a demonstration if students need more help seeing what is going on, or even let students work with colored ice cubes right from the start. If ice cubes and hence melt water are dyed right away, it becomes a lot easier to observe and deduct what is happening. Feel free to bring the photos or time lapse movie below as a backup, too!
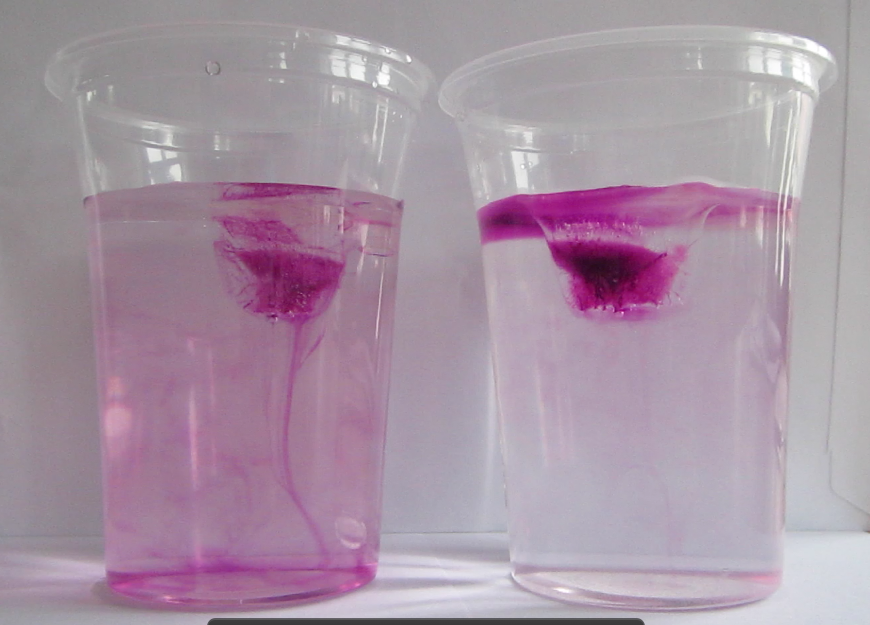
When the ice cubes start melting, it becomes very clear that they do so in different manners. In the left cup, the cold meltwater from the ice cube is denser than the lukewarm water in the cup. Hence it sinks to the bottom of the beaker and the water surrounding the ice cube is replaced by warmer water. On the right side, the lukewarm salt water is denser than the cold melt water, hence the cold meltwater floats on top, surrounding the ice cube which therefore melts more slowly than the one in the other cup.

The ice cube in the fresh water cup (left) is almost completely gone and the water column is fairly mixed with melt water having sunk to the bottom of the beaker. The ice cube in the salt water cup (right) is still a lot bigger and a clear stratification is visible with the dyed meltwater on top of the salt water.
And here a time-lapse movie of the experiment.
Another way to look at the experiment: With a thermal imaging camera!

Cold (dark purple) ice cubes held by warm (white-ish) fingers over room-temperature (orange) cups with water

After a while, both cups show very different temperature distributions. The left one is still room temperature(-ish) on top and very cold at the bottom. The other one is very cold on top and warmer below.
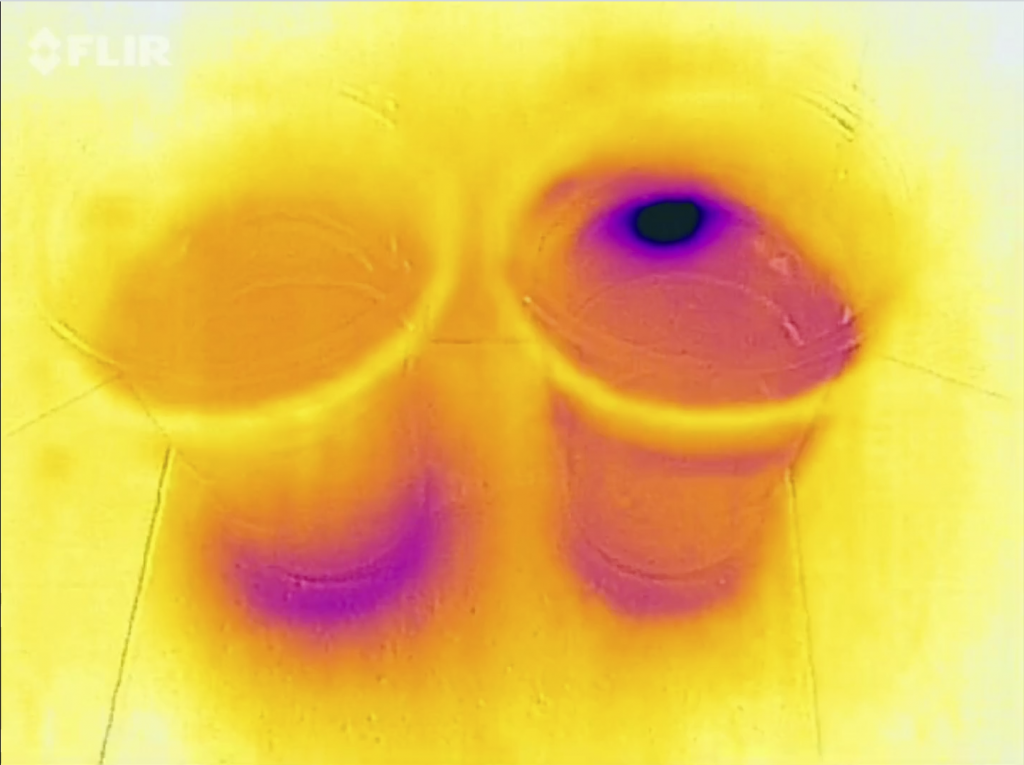
When you look in from the top, you see that in the left cup the ice has completely melted (and the melt water sunk to the bottom), whereas in the right cup there is still ice floating on top.
Assessment
Depending on the audience I use this experiment with, the learning goals are very different. Therefore, no one assessment strategy can be used for all different applications. Below, I am giving examples of what are possible ways to assess specific learning goals:
– Students apply the scientific process correctly: Look at how hypotheses are stated (“salt melts ice” is not a testable hypothesis, “similar-sized ice cubes will melt faster in salt water than in fresh water of the same temperature” is).
– Students are able to determine what kind of density-driven circulation will develop: Suggest modifications to the experiment (e.g. ice cubes are made from salt water, or ice cubes are held at the bottom of the cups while melting) and ask students to predict what the developing circulation will look like.
– Students can make the transfer from the flow field in the cup to the general ocean circulation: Let students compare the situation in the cup with different oceanic regions (the high Arctic, the Nordic Seas, …) and argue for which of those regions displays a similar circulation or what the differences are (in terms of salinity, temperature, and their influence on density).
In general, while students run the experiment, I walk around and listen to discussions or ask questions if students aren’t already discussing. Talking to students it becomes clear very quickly whether they understand the concept or not. Asking them to draw “what is happening in the cup” is a very useful indicator of how much they understand what is going on. If they draw something close to what is shown on slide 28 of the attached slide show, they have grasped the main points.
Equipment
Don’t worry, it is totally feasible to bring all the equipment you need with you to run the experiment anywhere you want. This is what we brought to EMSEA14 to run the workshop three times with 40 participants each:

What we brought to EMSEA14 to run workshops on the ice cubes melting in fresh and salt water experiment
In one big grocery bag:
- 4 ice cube trays
- 4 ice cube bags (backup)
- 2 thermos flasks (to store ice cubes)
- 1 insulating carrier bag (left)
- 4 empty 1.5l water bottles to mix & store salt water in
- 1 tea spoon for measuring salt
- 500g table salt
- 21 clear plastic cups for experiments
- 10 clear plastic cups to hand out ice cubes in
- 11 straws (as pipettes)
- 1 flask of food dye
- 11 little cups with lids to hand out food dye in
- nerves of steel (not shown :-))
And if you are my friend, you might also get the “ice cube special” — a pink bucket with all you will ever need to run the experiment! Below is what the ice cube experiment kit looks like that I made for Marisa, with labels and everything…
References and Resources
This activity has been discussed before, for example here:
- http://www.lawrencehallofscience.org/comsci/pdfs/2_Teaching_and_Learning.pdf
- http://www.usc.edu/org/cosee-west/glaciers/Ice_Cube_ExptFINAL.pdf
- http://www.cesn.org/cosee_CD/web/activity/Melting_Ice.pdf
I have also written about it a lot on my blog, see posts tagged “melting ice cubes experiment“.
—
P.S.: This text originally appeared on my website as a page. Due to upcoming restructuring of this website, I am reposting it as a blog post. This is the original version last modified on November 4th, 2015.
Melting ice cubes experiment — observing the finer details
If you don’t know my favourite experiment for practically all purposes yet (Introduction to experimenting? Check! Thermohaline circulation? Check! Lab safety? Check! Scientific process? Check! And the list goes on and on…), check it out here. (Seriously, of you don’t recognize the experiment from the picture below, you need to read up on it, it’s awesome! :-))
Susann and I got funding from PerLe (our university’s project to support teaching innovation) to add a couple of cool new features to Susann’s “intro to meteorology” lecture, and doing a hands-on experiment with 50 students in a lecture theatre in their second lecture was only one of the first of many more to come.

We used the experiment to introduce the students to oceanic circulation, and this experiment is, in my experience, very engaging and sparks curiosity, as well as being very nicely suited as a reminder that things are not as easy as they seem to be when you see those nice plots of the great conveyor belt and all the other simplified plots that you typically see in intro-level lectures. Especially understanding that there are many different processes at play simultaneously, and that they have different orders of magnitude and might act in different directions helps counteract the oversimplified views of the climate system that might otherwise be formed.

I usually use dye to make it easier to observe what’s going on in the experiment (either by freezing it directly into the ice cubes as shown in the picture on top of this blog post, or by dripping it onto the melting ice cubes when students have started to observe that — counter to their intuition — the ice cube in the fresh water cup is melting faster than the one in the salt water cup). We had dye at hand, but I decided on the spur of the moment to not use it, because the students were already focussing on other, more subtle, aspects that the dye would only distract from:
The shape of the ice cubes
In many of the student groups, the most prominent observation was that the shape of the melting ice cubes was very different in the fresh water and salt water case. In the fresh water case, the ice cube melted from the sides inwards: as a cylindrical shape with a radius that was decreasing over time, but in any instance more or less constant for all depths. In the salt water case, however, the ice cube melted upwards: The top did not melt very much at all, but the deeper down you looked the more was melting away. Why?
Condensation on the sides of the cup
Another observation that I prompted was in what regions the cups showed condensation. In the fresh water case, there was a little condensation going on everywhere below the water line, and sometimes there were vertical streaks down from where the ice cube was touching the wall. In the salt water case, there was only a small band of intense condensation close to the water level.
This, not surprisingly, looks very similar to what a thermal imaging camera sees when observing the experiment (as described in this post).

Taken together, those two observations are quite powerful in explaining what is going on, and it seemed to be a fun challenge for the students to figure out why there was condensation on the outside of the cups in the first place (does condensation occur in warmer or colder places?), what it meant that different places ended up being warmer or colder, and how all of that is connected to global ocean circulation. Definitely an experiment I would recommend you do! :-)
Teacher training at Lotseninsel
As I mentioned yesterday, I recently contributed to a teacher training on Lotseninsel, a tiny island on the Baltic Sea coast. The training was run by the Ozean:Labor of the Kieler Forschungswerkstatt, and we spent Friday to Sunday there. I’m going to show you some impressions of that weekend here.
At first, it did not look promising:

We had to pack A LOT of equipment on a small boat in pouring rain to bring everything over to the island.

After unpacking all that stuff, we went to test some instrumentation in the pouring rain. This is our cute ROV:

In the evening, when all the teachers had arrived, we started with the workshops and continued until late in the night. Below you see two groups of teachers working on 3×3 m stretches of the beach, collecting plastic to map the pollution of the beach.

The next day, the group was split up in two parallel groups. One doing stuff like this:



The other group, in which I was involved, doing stuff like this:

Obviously we had to do the melting ice cube experiment in a workshop on ocean physics!

But Johanna and Dennis did tons of other cool stuff, too, like for example this demonstration of salt inflow events into the Baltic Sea:

And again, the second time that same workshop was run in the afternoon for the second group of teachers. Amazing how quickly the weather changed!

But of course our group also did some field work: Water sampling and then analysing nutrients, salinity, oxygen concentration…

The next day, I got to see my first fish dissection. I know why I studied physics…

I am not showing you the gory pics here because that’s not what we do on this blog ;-)

Also really cool: Those are baleens, those filters that whales use! Never touched them before.

But we also got some time to enjoy the weather and play with our equipment: Those are Jeannine, Dennis and Johanna, who I had the pleasure to work with. It was great fun!

Even though the amazing weather only lasted for a short while, this is them arriving back on the main land with the last of three tours to shuttle everything back…

But I had a great weekend! And if you haven’t yet, go back and look at the lighthouse on Lotseninsel. I could spend years there, taking pictures from different angles and in different weathers… So pretty :-)
Melting ice cubes & thermal imaging camera
I haven’t talked about my favourite experiment in a long time (before using it last week in the MeerKlima congress and suddenly talking about it all the time again), because I felt like I had said everything there is to say (see a pretty comprehensive review here) BUT! a while back my colleagues started playing with a thermal imaging camera and that gave me so many new ideas! :-)
I showed you this picture yesterday already:

Here we see ice cubes melting in fresh water and salt water (and my very fancy experimental setup. But I am pretty proud of my thermal insulation!). Do you know which cup contains which?
Here are some more pics: The ice cubes before being dropped into the cups. Clearly dark purple is cold and yellow/white is warm (see my fingers?)

After a while (5ish minutes), the cold meltwater has filled up the bottom of the freshwater cup while floating on top of the salt water cup:

Looking in from the top, we see that the ice cube in salt water hasn’t melted yet, but that the other one is gone completely and all the cold water has sunk to the bottom of the beaker.
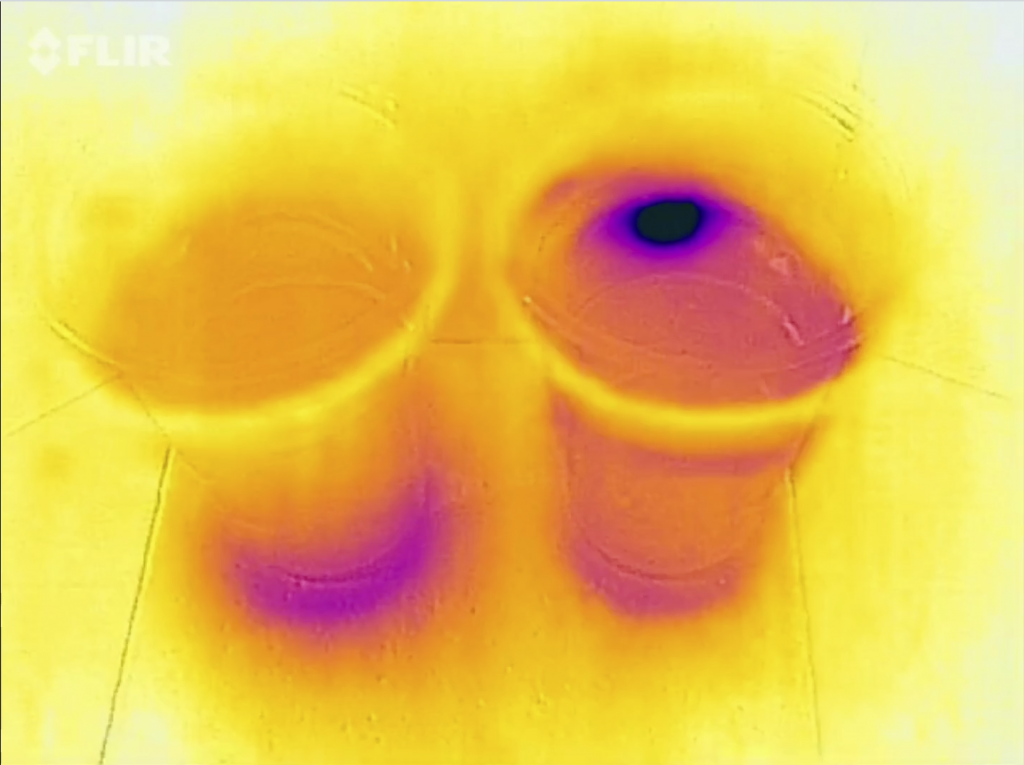
When you check out the movie at the bottom of this post, you will notice that this experiment doesn’t work quite as well as I had hoped: In the saltwater cup, the ice cube floats against the wall of the cup and for quite some time it looks like there is a plume of cold water sinking in the salt water. I’m not quite sure what’s going on there. If it’s showing up like that because the cup is such a good thermal conductor, then why is the “plume” directional and not spreading in all directions? If there really is a plume, then how did it get there? It shouldn’t be! So many questions!
There really can’t be a plume of cold melt water in the salt water cup. For my workshop last week I made the plot below (which, btw, I don’t think anyone understood. Note to myself: Explain better or get rid of it!). So unless the plume is cold salt water, there is no way anything would sink in the salt water cup.
So maybe we are cooling the salt water around the ice cube which then sinks and shows up because it is close to the wall of the cup? We can’t look “into” the cup with a thermal imaging camera, we can only see the surface of the cup (See, Joke? Maybe it is useful after all to learn all that stuff in theoretical oceanography ;-)). That’s also why we don’t see a plume of cold melt water in the freshwater case like we see when we have dyed ice cubes and see the melt water plume, like below:
Anyway. Here is the video, in which you sometimes see my finger, pushing the ice cube away from the beaker’s wall to finally get to a state that looks like what I wanted to show you above:
My workshop at MeerKlima.de
Today I ran a workshop at the MeerKlima.de congress in Hamburg: A congress for high school students, organised by a student committee. The large lecture theatre of the chemistry department at the University of Hamburg was crowded for the opening lecture by Mojib Latif:

For my workshop, however, we set a limit of 40 participants due to the size of the room (and the amount of stuff that I had lugged in from Kiel. Yesterday’s ice cubes did very well, btw!). And there were two TV crews and a photographer documenting the awesome ice cube experiment.


You can watch documentaries of the workshop here and here (both in german).
Sneak peak of those two documentaries, obviously only of the tiny little sequences featuring me:


And thanks to Johanna and Dirk for their support before, during and after the workshop!
I also got to watch another workshop by a colleague, who used the Monash Simple Climate Model (which I have talked about here) and I have got to say: That is such an awesome tool for teaching about models and/or the climate system! You will definitely hear more about it in the future as I incorporate it into my own teaching.

And last not least we had a phone call to the Meteor off Peru which rounded off a day full of bumping into people I hadn’t seen in a while. Always great to reconnect with old friends and colleagues!

It was great fun to be part of this congress, and it was a great way to experience first hand how science outreach can work in such a format. Since the congress was curated by the students themselves, many students were very interested and asked great questions. Also, the topics of the workshops corresponded closely to what students really wanted to see and hear. It would be amazing to see this scaled up next year, maybe over several days and with more parallel sessions, so that participating students really get to pick and choose exactly what topic they are interested in and that even more students get the opportunity to experience such an amazing congress!
Workshop prep and a riddle
Looking at the picture below, can you guess which experiment I am going to do at the MeerKlima.de workshop? Yep, my favourite experiment — melting ice cubes! :-)
And I am obviously prepared for several extensions of the classic experiment should the students be so inclined…
Now I only need to get the ice cubes from Kiel to Hamburg — and as ice cubes, not a colourful, salty, wet mess :-)
Having gotten that backstory as a hint, any idea what’s going on with the spoons below?
Yep. Freshwater on the left, salt water on the right. Different refraction indices due to different densities. Neat :-)