First of all, let me say how much I love having chats like the one Elin and I had over the weekend (which you only see the very beginning of above). I had gotten into a bit of a rut kitchen oceanography-wise, which, I am happy to report, is over now! Thanks, Elin! :-)

One of my favourite experiments of all times are the ice cubes melting in fresh water and salt water. I’ve written about this experiment many times before, so if you aren’t familiar with it, check out my introductory post or all the posts related to the original experiment, because now we are going to take it one step further.
Ready?
Last year, Elin told me about a conversation she had had with Prof. Emeritus Arne Foldvik (our hero when it comes to tank experiments!) about someone considering towing icebergs from Antarctica to some tropical place to use as freshwater supply. Arne mentioned that when the water in which the iceberg swims gets warmer than 27°C, the situation changes, as in that the iceberg’s melt water now is denser than the warm saltwater it is swimming in. So the assumption from that would be that the melt water would sink, rather than form a layer floating around the iceberg.
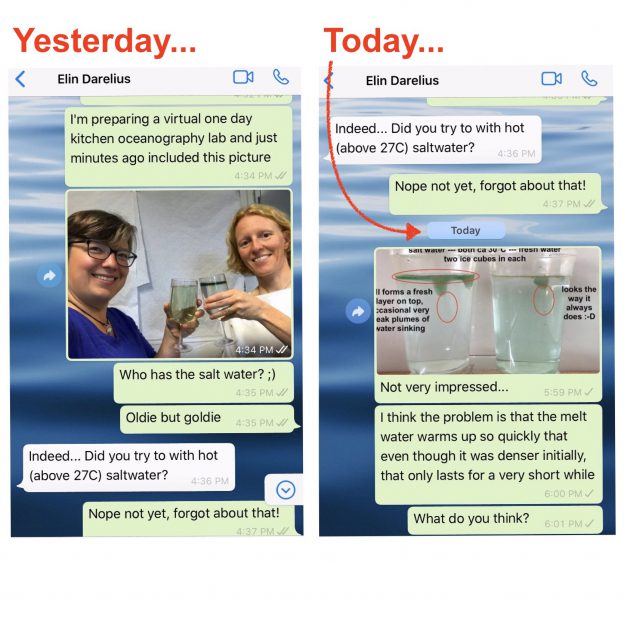
And that’s the experiment I had been wanting to try and only got around to doing when Elin reminded me on Saturday. Results were … a bit disappointing. At least at first:

So what is happening is that even though the melt water is initially denser than the salt water, it doesn’t stay that way for very long, because diffusion of temperature is very fast and the fresh meltwater plumes don’t have to warm up by very much before they are less dense than the warm saltwater, so that happens very quickly.
In the movie below we see evidence of this: Around minute 1 (marked in the video) we can spot plumes of dense water sinking down, but at the same time we very clearly see a (green) freshwater layer forming on top of the salt water.
(The “Happy Birthday Arne” in this movie refers to Arne Foldvik’s 90th Birthday which was yesterday!)
For the experiment, I kinda eyeballed the salinity and also my thermometers might not be the most suitable choice for measuring water temperatures, at least in that temperature range. But as Elin and I discussed as the chat above went on: I think it won’t make a big difference to fiddle with temperatures and salinities, in the end the dominant process will be the molecular diffusion of heat that will always quickly warm the meltwater, making it buoyant. And I actually think that this makes this experiment even more interesting — to show how different processes are acting at the same time, and it’s not always obvious right away which one will be the most important one. Kinda similar to what I showed in yesterday’s post: molecular diffusion of heat will sneak up on you faster than you think :-)
Would the same thing also happen if we didn’t just have small ice cubes with low meltwater “production”, but icebergs, where the meltwater plumes would have a larger volume and so wouldn’t be warmed up as easily? Who knows… But my kitchen is too small to try and I’m too lazy to do the maths, so now it’s your turn! :-)