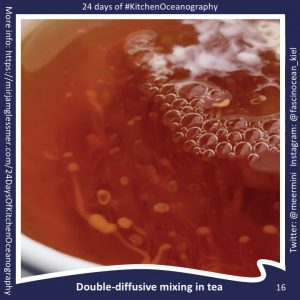
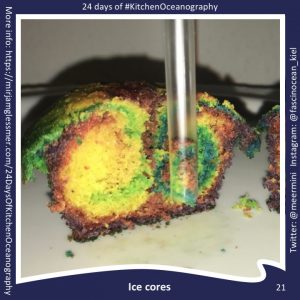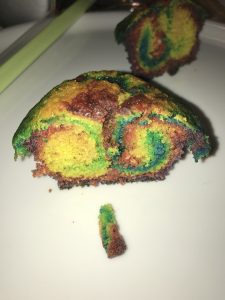#KitchenOceanography
/ˈkɪtʃɪn ˌəʊʃəˈnɒɡrəfi/ noun Experimenting with ocean physics using only household items Observing oceanographic processes in, or during the preparation of, food and drinks DIYnamics: Rotating tank experiments based on LEGO…