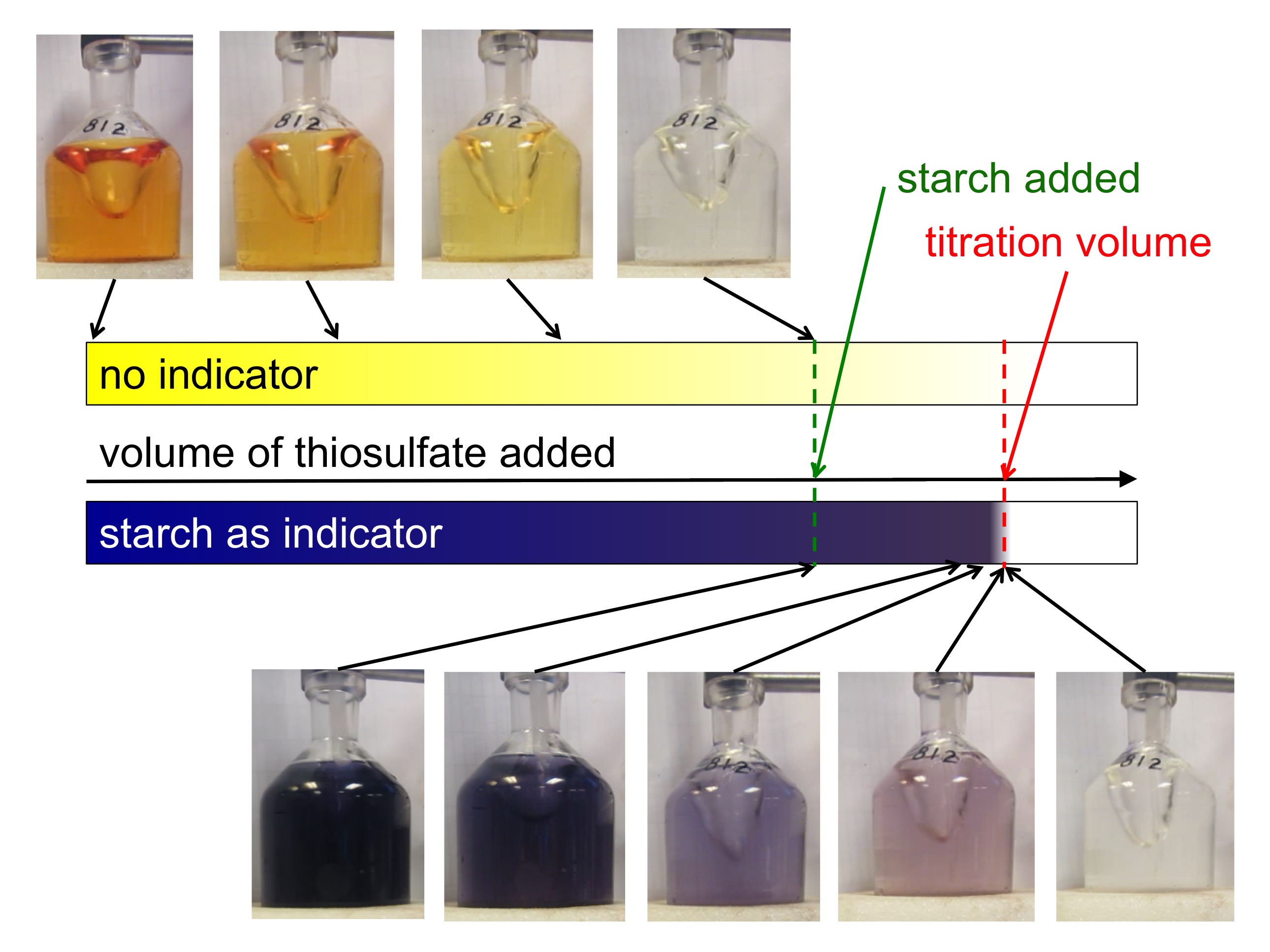
So now we have prepared our sea water sample and are ready to start titrating to figure out the concentration of dissolved oxygen. The sample itself changes color with added thiosulfate, it goes from yellow to lighter yellow to clear over a wide range of added thiosulfate. But determining the titration volume just based on this is a pain.
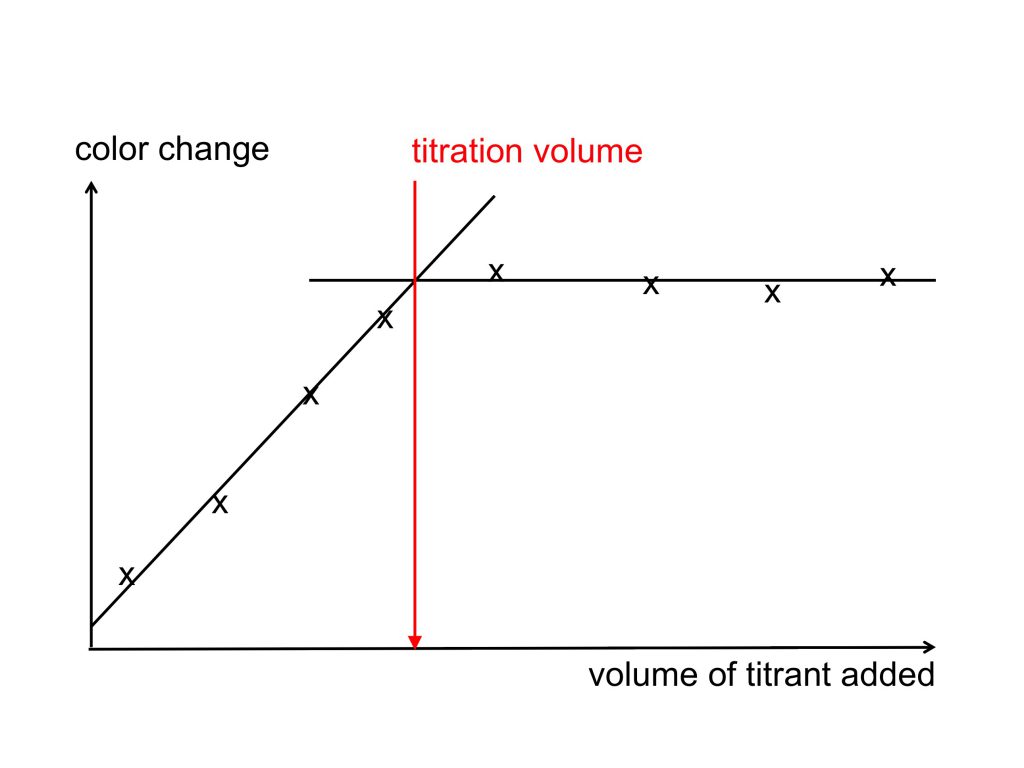
When measuring dissolved oxygen with automatic titration, the instrumentation that I used previously used a UV lamp and a detector: After each tiny volume of the titrant was added, the amount of UV light that made it through the sample as it changed it color from its original apple juicy-color to clear was measured and noted. Two lines were fitted to those data points: One while the color of the sample was still changing, the other when it wasn’t any more. The titration volume is found at the intersection of those lines.
When measuring oxygen with manual titration, like I did on this cruise, we can’t take all those individual data points and then fit lines, we can just take one single reading the moment we think the titration volume has been reached (well, we can note down volumes when we think we are close, and then just use the one we think was closest to something actually happening. But we don’t have a good account of how close we were at each of those volumes, and we can’t go back in time to compare values, so it still comes down to either getting it right or not). Having a good indicator that clearly shows when the titration volume (I.e. the point at which the amount of thiosulfate solution added to the sample is proportional to the concentration of dissolved oxygen in the original sample) is reached is key.
Luckily, Kristin prepared an awesome starch mixture which I got to use that makes it a lot easier to determine the point when titration is done. You add it when the yellow of the sample has become so light that it gets difficult to see whether it is still yellow or clear already, and the sample turns a deep, dark purple. As you come closer to the titration point, color changes little until you are very close, when it changes very rapidly (that’s why you only put it in once you are fairly close, otherwise the looooong time with no changes would likely lead to you becoming too impatient, adding too large volumes at a time, and over-titrating [at least if you are like me at all]). Adding starch late and then having it change very sensitively to added thiosulfate makes it very easy to determine the exact volume of thiosulfate needed.
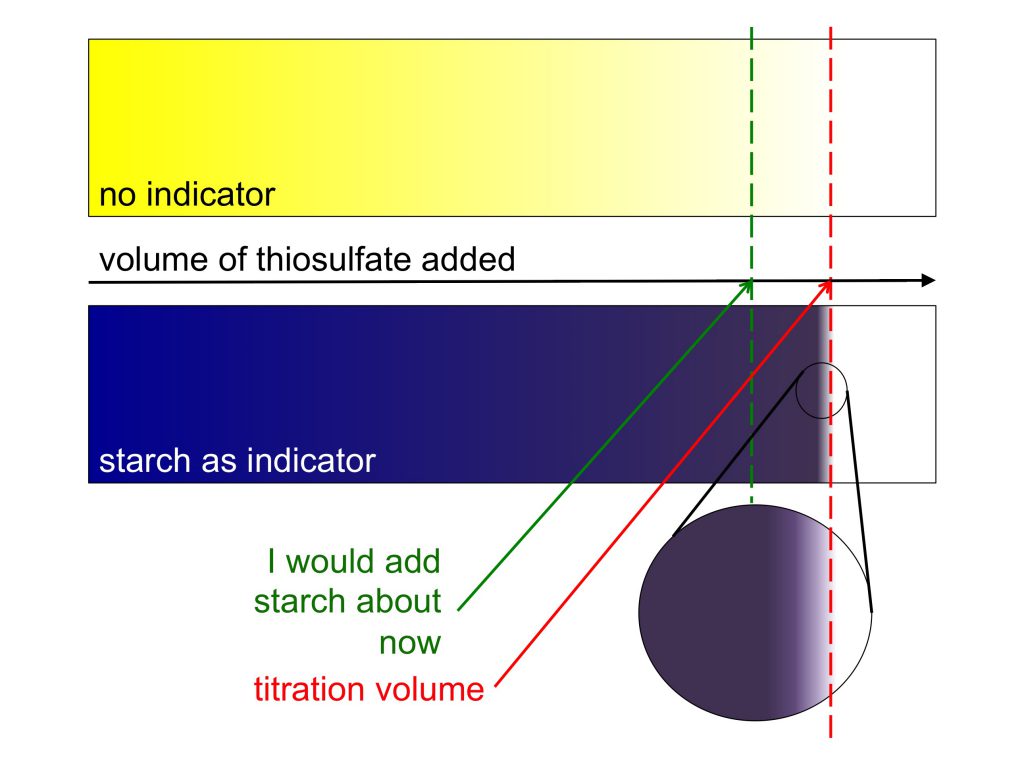
Winkler titration: Sketch of the color changes of just the sample, or a sample with an added starch solution, during titration. Note how it is a lot easier to find the titration volume when starch is added at the right moment!
And here are a couple of impressions of what it looks like for real:
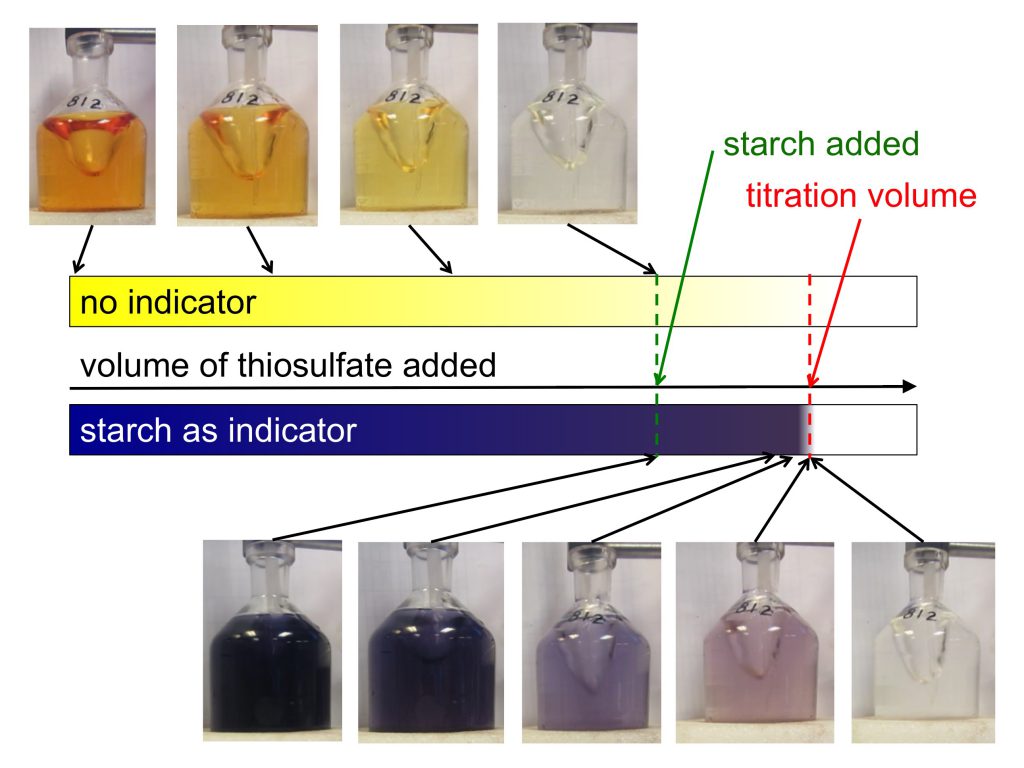
Winkler titration: Sketch + photos of the color changes of just the sample, or a sample with an added starch solution, during titration. Note how it is a lot easier to find the titration volume when starch is added at the right moment!
When writing this post and showing it to people, I have been warned repeatedly to not make myself redundant by making it too easy for other people to just print this blog post and go take my spot on the next cruise to measure oxygen. So I just want to state: Clearly there is more to measuring oxygen than what was shown here! For example, you need to measure standards to calibrate your measurements, which I am too lazy to write about right now. And most importantly: If something goes wrong, you need to be able to figure out how to fix things. And that’s not always a piece of cake, I can tell you… So please don’t use this as a manual. But I’m happy to talk about my experiences if anyone is interested!
And Kjetil and Emil, I really want to go on that winter cruise! :-)