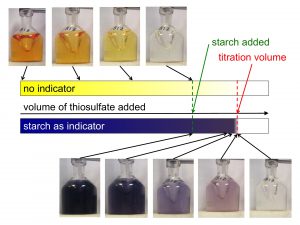
Measuring the concentration of dissolved oxygen in sea water – Part 3 of 3 – finding the titration volume by looking at the change in color
So now we have prepared our sea water sample and are ready to start titrating to figure out the concentration of dissolved oxygen. The sample itself changes color with added…
