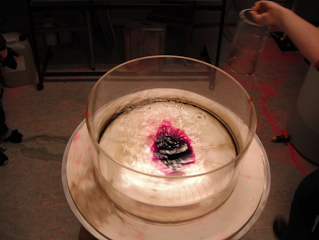
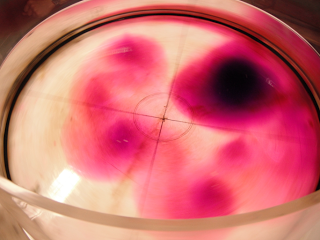
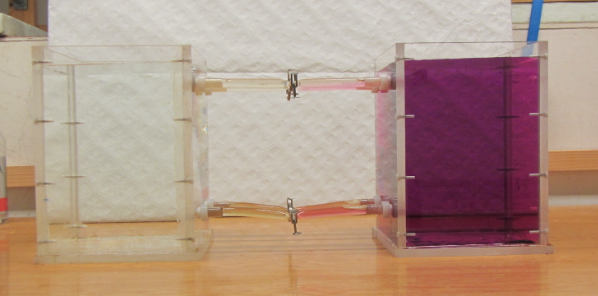
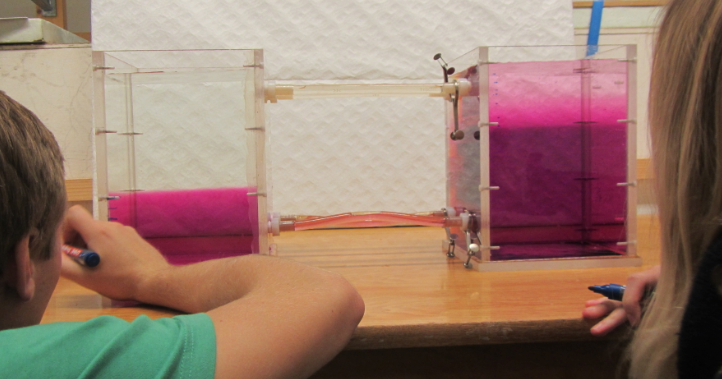
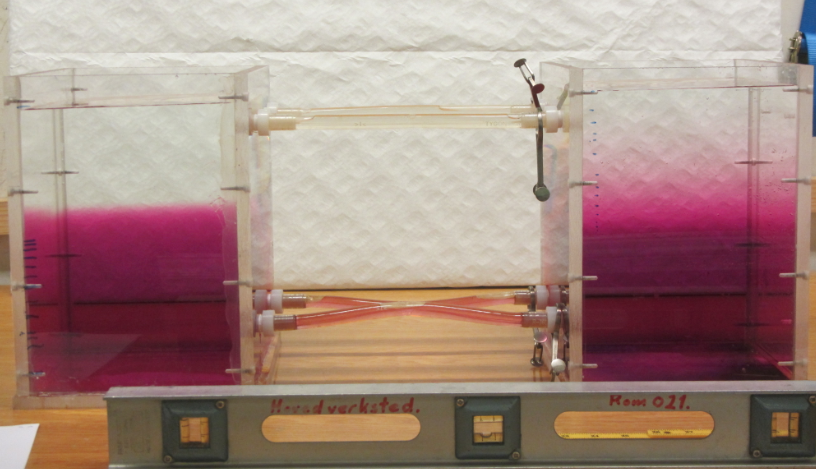
Removing a barrier between waters of different densities and watching what happens. (deutscher Text unten)
Today, one of the groups performed a classical experiment (shown for example here) – but the awesome thing is that they came up with the planning pretty much by themselves in order to determine the effects of temperature and salinity on density. They compared water of the same temperature, but one fresh and one salty; warm salty vs cold fresh water; and cold salty vs warm fresh water. They predicted the outcome correctly, and we are showing two movies below: One normal movie and one in slow motion. Enjoy!
Heute hat eine Gruppe ein klassisches Experiment reproduziert. Allerdings haben sie es quasi selbstständig entwickelt.
Um den Effekt von Temperatur und Salzgehalt auf die Dichte zu bestimmen, werden zwei Wassermassen in einen Tank gefüllt, durch ein Wehr getrennt. Das Wehr wird herausgezogen und die dichtere Wassermasse schichtet sich unter die weniger dichte. Die Gruppe hat drei Fälle verglichen: Wasser gleicher Temperatur mit und ohne Salz; warmes salziges Wasser mit kaltem süßen; und warmes süßes Wasser mit kaltem salzigen. Der Film unten zeigt eine Zeitlupe der Bewegung.
:-)