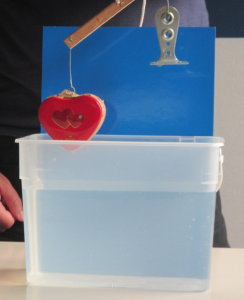
There are a lot of misconceptions related to hydrostatic pressure. One of them is that if you took a jug like the one below (or a U-tube, as in my post on letter tubes and misconceptions around hydrostatic pressure) the water level would have to be higher in the narrow snout of the jug than in the main body. So when I saw a cheap-ish fat separator jug recently, I had to get it “for my blog” (ok, because I wanted to play with it) to show that water, indeed, seeks its level.
But it turns out it is really difficult to take pictures of the water level! My first attempt (above) was with dyed water because I thought that might make it easier to see what is going on. Turns out that the adhesion of water makes it really difficult to observe the water level: The water is pulled up along the walls of the jug, leading to these weird changes in color.
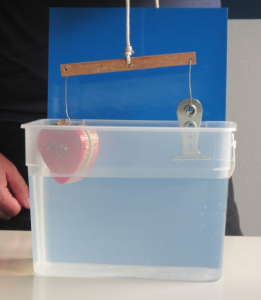
In the picture below, taken from slightly above water level, you can see the curvature of the water surface both in the main body of the jug and in the spout:
Using clear water turns out to be the best way to photograph this phenomenon (below).
So there you see it: Water seeks its level!
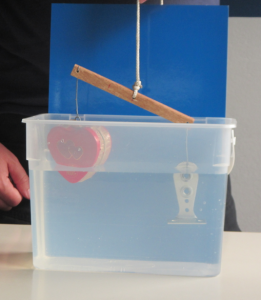
Another problem with this setup is that the spout is so narrow that I am not entirely sure capillary effects don’t come into play.
One thing we can do about it: reduce surface tension by adding a little bit of dish soap!
Now you clearly see it. Don’t you? :-)







 Now. Ice cubes on the “cold” end. For convenience, they have been dyed blue so that the cold melt water is easily identifiable as cold.
Now. Ice cubes on the “cold” end. For convenience, they have been dyed blue so that the cold melt water is easily identifiable as cold. A very easy way to get a nice stratification! And as you see in the video below, awesome internal waves on the interface, too.
A very easy way to get a nice stratification! And as you see in the video below, awesome internal waves on the interface, too.