Yep, I’ve been playing this weekend :-) After seeing this on Facebook a while back I just couldn’t resist… Enjoy! :-)
Tag Archives: kitchen oceanography
Evaporating sea water
How much salt is there in sea water? What concentration do you need before crystals start forming? What will those crystals look like? I am sure those are the kind of questions that keep you awake at night!
Of course this can easily assessed experimentally. On a visit to the University of Bergen’s Centre for Science Education just now, I was shown the result of such an experiment: A litre of water was mixed with 35 grams of salt to simulate sea water with its typical salinity. Below, you see what the beaker looked like after sitting out for three months.
You can see that salt crystals are forming at the walls of the beaker, but that their structure depends on depth below the initial water level (see the 1000 ml mark on the beaker).
When there is still a lot of water in the beaker, crystals look like ornate flowers. Then, the less water is left in the beaker, the more square the crystals become. And at the bottom of the beaker, you see the typical salt crystals you would expect.
Actually, even though they look like the kind of salt crystals I would expect, apparently someone who knows about crystallography commented that there must be other stuff in there than just cooking salt since the crystals don’t look the way they should. I need to read up on this! :-)
Anyway, this is an experiment that I want to do myself, so maybe in three months time there will be more pictures of this!
Thanks for a very nice lunch, Olaug, Frede, Andreas, Morven and Elin! Looking forward to working with you a lot more in the future! :-)
P.S.: with this blog post I am testing to blog pretty much “real time” from my mobile phone, so if you notice anything odd, please let me know!
One glance — do you know which of the bottles is empty?
The other day I was sitting in my conservatory with a friend when I had to take the photo below:
Can you see how one bottle refracts light and the other one does not? What does this tell us about whether there is water in either of those bottles? I met most “normal” people wouldn’t even notice a difference.
I know, I’m a nerd, but I have so much fun “discovering” small stuff like that! :-)
Frost flowers on ice cream: When you start thinking about phenomena and something really annoying, all of a sudden, becomes really cool.
Frost flowers on ice cream. You must have seen them before: They sometimes occur when you’ve had some ice cream, put the left-overs back in the freezer, and take them out again. And there you have it: Water-ice crystals all over your lovely ice cream! Completely annoying because, obviously, they only taste like water and mess up your whole ice cream experience (or is that only me)?
You know I’m kinda fascinated with ice crystals on frozen blended strawberries, but last time I had some, there weren’t only crystalline structures, but there was frost on it:
Frost occurs when water vapour freezes without going through the liquid phase. Look at the awesome crystals!
Once I started thinking about the process that formed the ice and realised that those were actually frost and not just ordinary ice crystals, they all of a sudden stopped being annoying and instead became something that I kinda look forward to finding when I open a tub of my frozen blended strawberries. Because the structures are different every time, and really really pretty! And also how awesome is it to know that those ice crystals formed from water that wasn’t even liquid? Yes, this is the kind of stuff that makes me happy! :-)
Taking the hydrostatic paradox to the next (water) level
How well do people understand hydrostatics? I am preparing a workshop for tomorrow night and I am getting very bored by the questions that I have been using to introduce clickers for quite a lot of workshops now. So I decided to use the hydrostatic paradox this time around.
The first question is the standard one: If you have a U-tube and water level is given on one side, then what is the water level like on the other side? We all know the typical student answer (that typically 25% of the students are convinced of!): On the wider side the water level has to be lower since a larger volume of water is heavier than the smaller volume on the other side.
Clearly, this is not the case:
However, what happens if you use that fat separator jug the way it was intended to be used and fill it with two layers of different density (which is really what it is intended for: to separate fat from gravy! Your classical 2-layer system)?
Turns out that now the two water levels in the main body of the jug and in the spout are not the same any more: Since we filled the dense water in through the spout, the spout is filled with dense water, as is the bottom part of the jug. Only the upper part of the jug now contains fresh water.
The difference in height is only maybe a millimetre, but it is there, and it is clearly visible:

Water level 1 (red line) is the “main” water level, water level 2 (green line) is the water level in the spout and clearly different from 1, and water level 3 is the density interface.
We’ll see how well they’ll do tomorrow when I only give them levels 1 and 3, and ask them to put level 2 in. Obviously we are taking the hydrostatic paradox to the next (water) level here! :-)
A string of bubbles
Have you ever noticed champagne bubbles that form as a string right in the middle of the glass and hardly anywhere else? This leads to the very cool pattern you see here:
Astrid and I recently happened to notice how differently bubbles in champagne and in mineral water behaved. In the mineral water, bubbles formed in random spots along the sides of the glass. In the champagne, they mainly formed in the middle; and formed a string of rapidly forming bubbles.
So now I was hoping for a really interesting explanation of why the bubbles behave so differently. They form at different rates, but that makes sense if the partial pressure of CO2 in both drinks is different. After a bit of research on the web it turns out that fancy champagne glasses have tiny scratches right in the center of the glass to serve as condensation nuclei — in other words: to cause exactly what we observed: A nice string of pearls instead of bubbles forming randomly along the sides of the glass. So theoretically, if we had had our mineral water from the same glasses, we would have observed the same thing in mineral water. What a disappointing explanation!
[vimeo 146250051]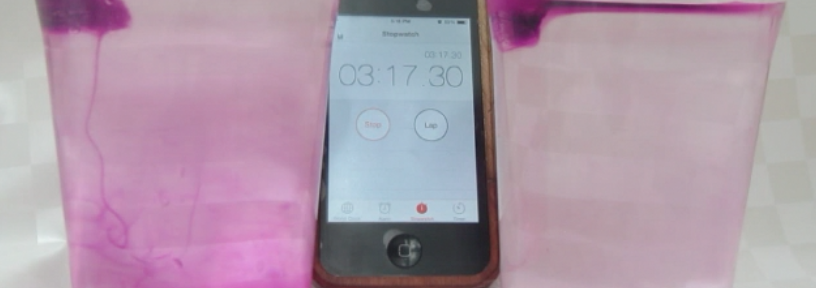
Considered exemplary: My “ice cubes melting in fresh water and salt water” in the “on the cutting edge” teaching collection! :-)
After reading recently that I am being considered the queen of the melting ice cube (aaaaw, thank you!!!), having my movies of the experiment featured in Elin Darelius and Petra Langebroek’s article on “fun in the kitchen”, and hearing that the activity I posted on the “On The Cutting Edge”‘s website using the very same experiment has been awarded “exemplary” status (so excited!), I simply had to talk about this experiment again. Even though just two days ago I talked about using it as a tool to let future instructors experience inquiry-based learning. One really cannot talk about this experiment enough!
Go check it out on On The Cutting Edge’s website, check out my own summary page with all the best tips and tricks, watch the movie below or look at all the posts tagged with “melting ice cubes experiment“. And then go and DO THE EXPERIMENT!
Using the “melting ice cube” experiment to let future instructors experience inquiry-based learning.
Using the “melting ice cube” experiment to let future instructors experience inquiry-based learning.
I recently (well, last year, but you know…) got the chance to fill in for a colleague and teach part of a workshop that prepares teaching staff for using inquiry-based learning in their own teaching. My part was to bring in an experiment and have the future instructors experience inquiry-based learning first hand.
So obviously I brought the ice cubes melting in fresh water and salt water experiment! (Check out that post to read my write-up of many different ways this experiment can be used, and what people can learn doing it). On that occasion the most interesting thing for me was that when we talked about why one could use this — or a similar — experiment in teaching, people mainly focussed on the group aspect of doing this experiment: How people had to work together in a team, agree to use the same language and notation (writing “density of water at temperature zero degree Celsius” in some short syntax is not easy when you are not an oceanographer!).
And this experiment never fails to deliver:
- you can be 100% sure that at least in one group, someone will say “oh wait, which was the salt water again?” which hands you on a plate the opportunity to say “see — this is a great experiment to use when talking about why we need to write good documentation already while we are doing the experiment!”
- you can also be 100% sure that in that group, someone will taste the water to make sure they know which cup contains the salt water. Which lets you say your “see — perfect experiment to talk about lab safety stuff! Never ever put things in your mouth in a lab!”
- you can also be sure, that people come up with new experiments they want to try. At EMSEA14, people asked what would happen if the ice cubes were at the bottom of the beaker. Today, people asked what the dye would do if there was no ice in the cups, just salt water and fresh water. Perfect opportunity to say “try! Then you’ll know! And btw — isn’t this experiment perfect to inspire the spirit of research (or however you would say that in English – “Forschergeist” is what I mean!). This is what you see in the pictures in this blog post.
So yeah. Still one of my favorite experiments, and I LOVE watching people discover the fascination of a little water, ice, salt and food dye :-)
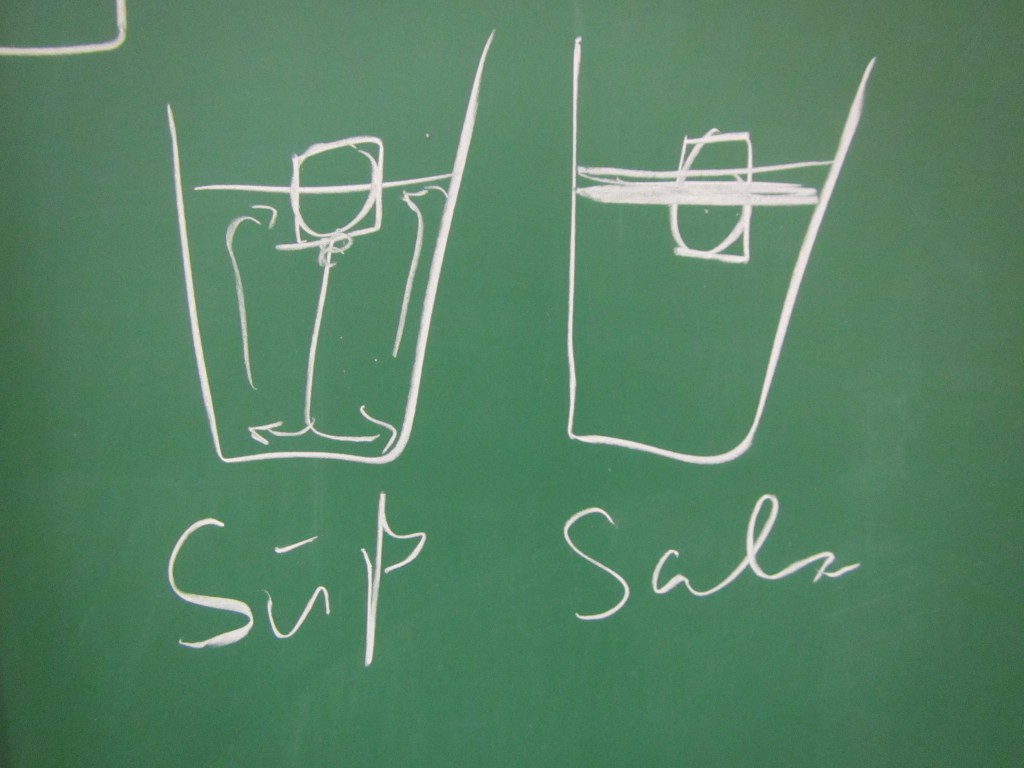
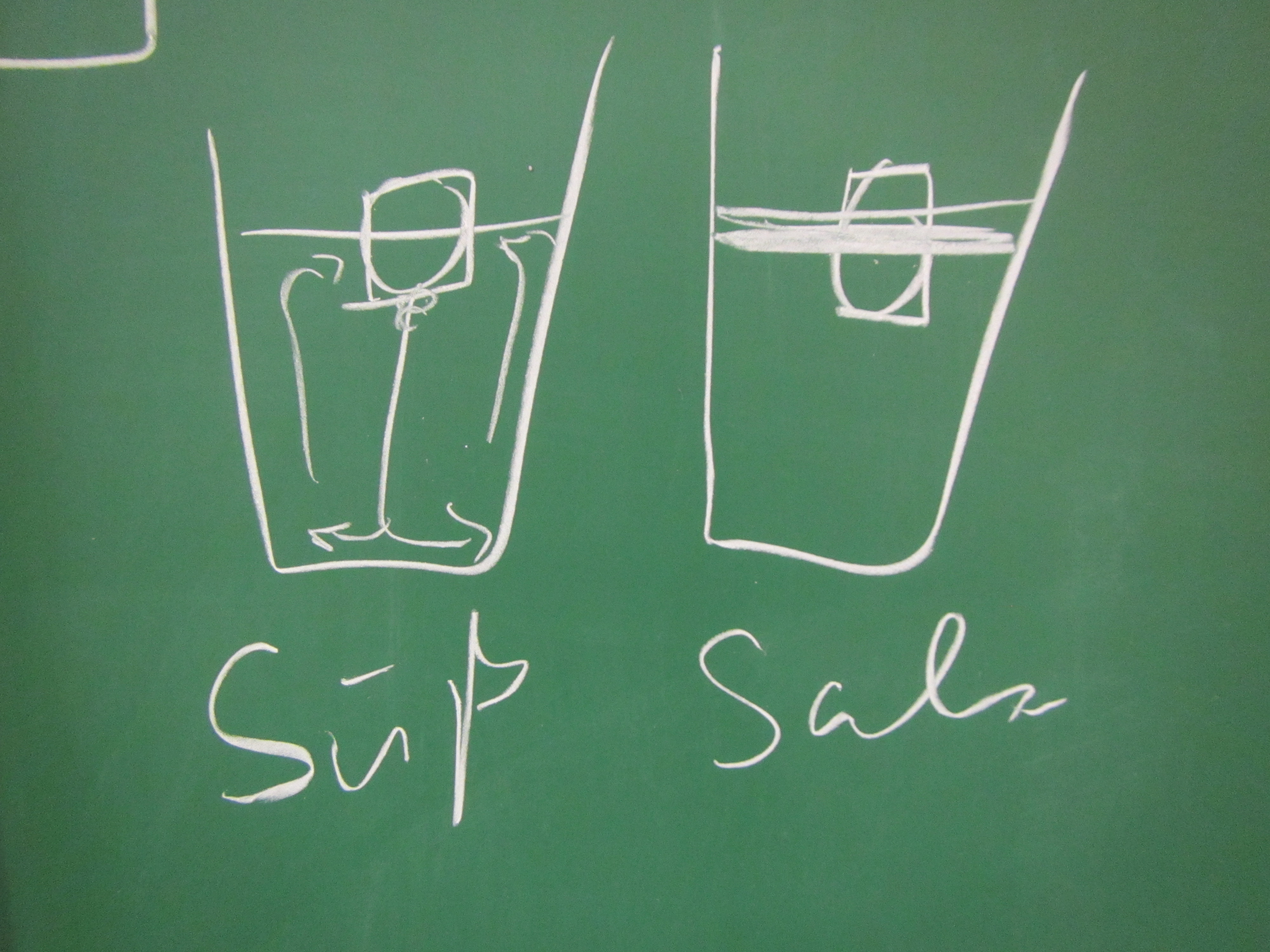
Btw, when I gave a workshop on active learning last week and mentioned this experiment, people got really really hooked, too, so I’ll leave you with a drawing that I liked:
One of the most exciting things about work travel?
One of the most exciting things about work travel? Staying in tons of different hotels, which all have different opportunities to play with water.
For example at a recent team event, there was this tap with a really efficient aerator, that made the hydraulic jump look even more exciting than usual:
And then at a conference last week, this happened:
Can you see what happened? Obviously, I turned the water on, and the right side of the armature fogged up because of all the cold water going through! (Even though I can assure you: My shower was nice and warm!)
And I am not even going to apologise for how excited I get by observing these kinds of things. Remember the kind of tap I have at home?
Still the coolest tap I have ever seen! :-)
My favorite demonstration of the coolest mixing process: Salt fingering!
I am updating many of my old posts on experiments and combining multiple posts on the same topic to come up with a state-of-the-art post, so you can always find the best materials on here. And today I would like to present you my favorite experiment: Salt fingering!
Check out the new page I made for salt fingering!
As you guys might have noticed, I’ve been playing around with my site a quite bit. My blog has moved to mirjamglessmer.com/blog in order to make room for static pages of my favorite experiments or teaching tips right at the landing site mirjamglessmer.com. What do you think? Good idea? Did you notice anything that isn’t quite working yet or do you have advice or wishes? Let me know!