Frost flowers! I learned about those in the context of Arctic and Antarctic ice formation. I kinda assumed that ice flowers only formed in salt water, because I remember hearing about how the ice needles that form wick up brine and that, due to their large surface (which you will remember noticing in the last post where we looked at them forming on trees), they are super important in the air-sea exchange of all kinds of stuff, like for example bromine. So imagine my excitement when I saw them growing the other day!

Frozen Schlei river in Schleswig
Frost flowers are really pretty by themselves, but they also tell us a lot about recent weather conditions. For example, they only form when the air is A LOT colder than the water/ice surface. Do you know the snowy ice crystals you sometimes find on the inside of ice cream containers when you’ve opened and refrozen them? Yep – same thing! I even suspect that the ice crystals I was talking about in this post are also frost flowers.

Frost flowers
I find it really fascinating how they are distributed over the larger surface of the Schlei river.

Schlei river in Schleswig coated in frost flowers
Here, for example, you see them forming on the edges of ice that has been broken up by some mechanical process. Judging from their placement, I would suspect that they only formed after the ice was broken and some of the pieces tilted up.

Cracked ice and frost flowers
Here, they were probably everywhere, but then the ice got broken up and some parts submerged. When the water there refroze, no snow flowers formed, just “normal” ice. However, the existing snow flowers seem to have continued growing!

Ice with frost flowers. Partially submerged and then refrozen into “normal” ice
The really interesting thing is that frost flowers don’t actually form from the water that is freezing below, but from water vapour in the air. Which, btw, explains why they can form on benches, ice cream lids or trees (all of which would be really difficult if they could only form on open water surfaces).

Ice with frost flowers. Partially submerged and then refrozen into “normal” ice
Above you see a larger part of the Schlei’s surface: Seems like there used to be frost flowers everywhere, but when the ice broke up, some of it got pushed out of the water, and as such preserving the frost flowers and letting them continue to grow. Meanwhile, other parts got flooded and only normal ice formed there. Maybe because the temperature gradient at that point wasn’t large enough any more?
Isn’t this just beautiful??? I could watch this all day, every day.

Frozen Schlei river in Schleswig with frost flowers
But let’s look at some more details. No idea why that patch of frost flowers formed there! But they seem to always start in small patches, which eventually grow together if the conditions are stable enough over long enough periods of time.

Frost flowers on ice
Here, we see the opposite situation to the one a couple of pictures up: “Normal” ice had formed, and then was broken up. And then, when the crack froze over, frost flowers formed!

Frost flowers growing in a crack in the ice
Very cool stuff!

Frost flowers
Yep, I would still just sit there and watch!

Frozen Schlei river in Schleswig




















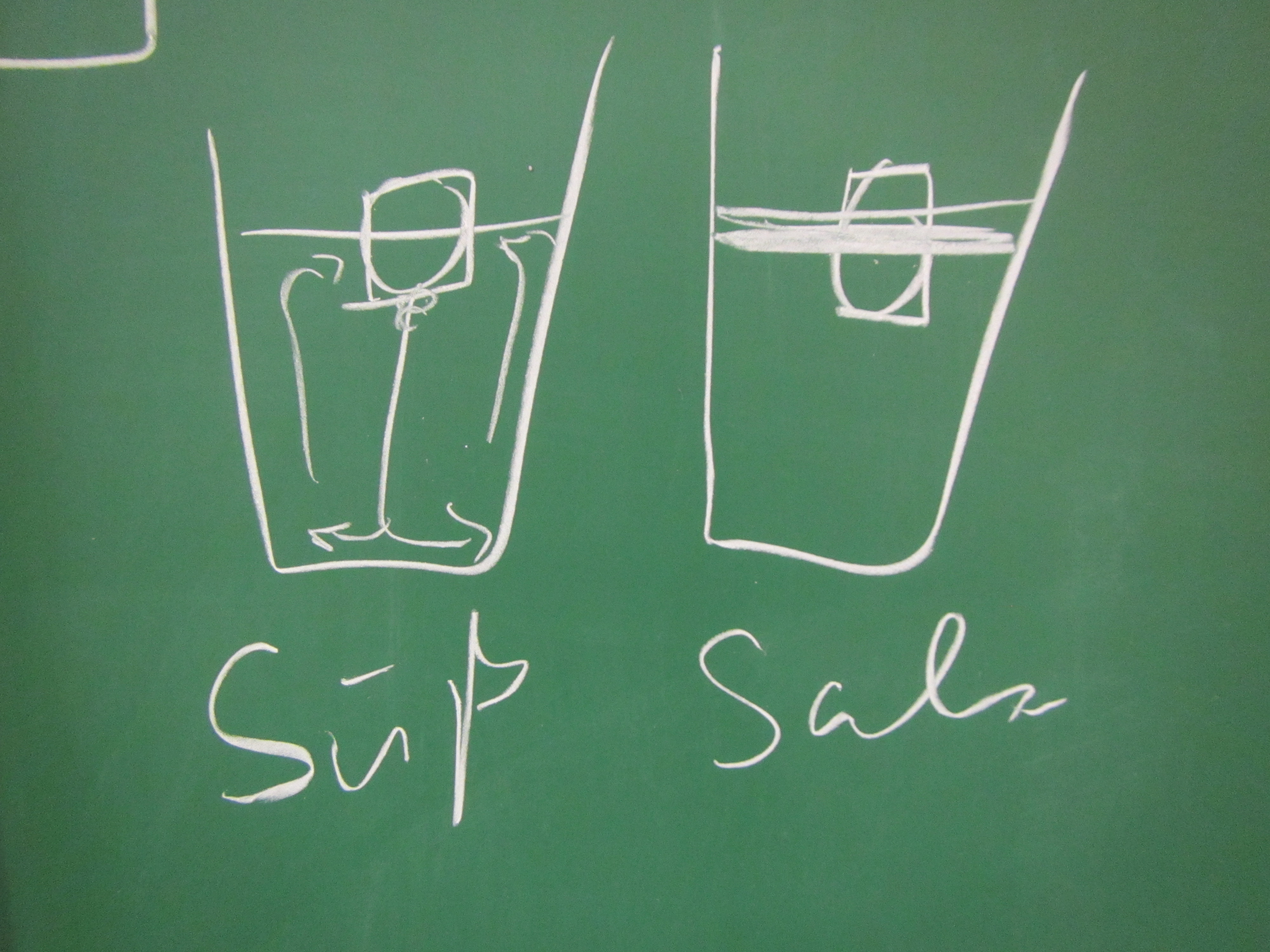




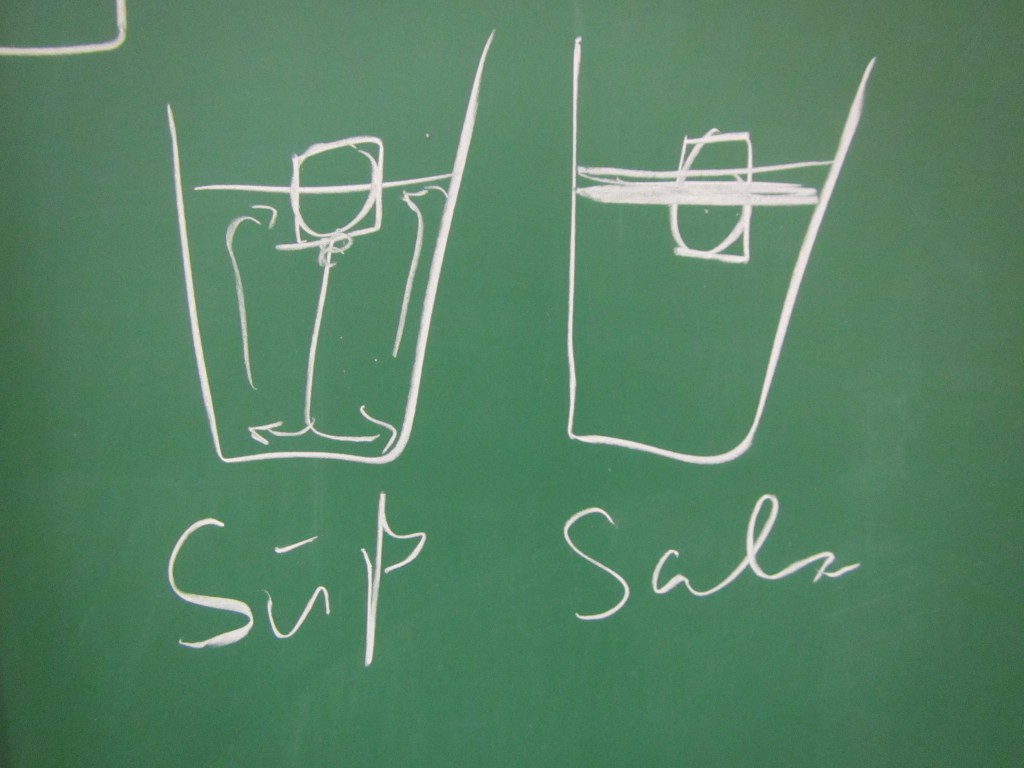


























 Now. Ice cubes on the “cold” end. For convenience, they have been dyed blue so that the cold melt water is easily identifiable as cold.
Now. Ice cubes on the “cold” end. For convenience, they have been dyed blue so that the cold melt water is easily identifiable as cold. A very easy way to get a nice stratification! And as you see in the video below, awesome internal waves on the interface, too.
A very easy way to get a nice stratification! And as you see in the video below, awesome internal waves on the interface, too.
