
Tag Archives: density


24 Days of #KitchenOceanography — Double-diffusive mixing

24 Days of #KitchenOceanography — Mediterranean Outflow

24 Days of #KitchenOceanography — Surface waves and internal waves

24 Days of #KitchenOceanography — Overflow experiment

24 Days of #KitchenOceanography — Melting ice cubes!
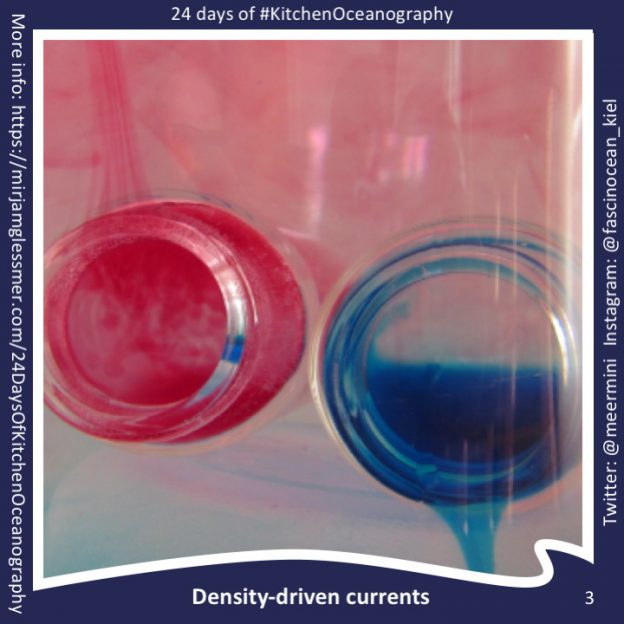
24 Days of #KitchenOceanography — Density-driven currents
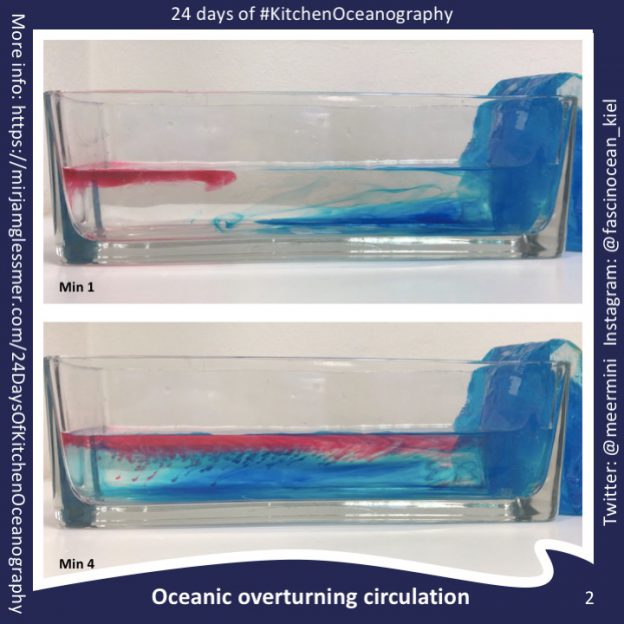
24 Days of #KitchenOceanography — Oceanic overturning circulation

24 Days of #KitchenOceanography — Layers in the ocean
Thermal forcing vs rotation tank experiments in more detail than you ever wanted to know
This is the long version of the two full “low latitude, laminar, tropical Hadley circulation” and “baroclinic instability, eddying, extra-tropical circulation” experiments. A much shorter version (that also includes the end cases “no rotation” and “no thermal forcing”) can be found here.
Several of my friends were planning on teaching with DIYnamics rotating tables right now. Unfortunately, that’s currently impossible. Fortunately, though, I have one at home and enjoy playing with it enough that I’m
- Playing with it
- Making videos of me playing with it
- Putting the videos on the internet
- Going to do video calls with my friends’ classes, so that the students can at least “remote control” the hands-on experiments they were supposed to be doing themselves.
Here is me introducing the setup:
Today, I want to share a video I filmed on thermal forcing vs rotation. To be clear: This is not a polished, stand-alone teaching video. It’s me rambling while playing. It’s supposed to give students an initial idea of an experiment we’ll be doing together during a video call, and that they’ll be discussing in much more depth in class. It’s also meant to prepare them for more “polished” videos, which are sometimes so polished that it’s hard to actually see what’s going on. If everything looks too perfect it almost looks unreal, know what I mean? Anyway, this is as authentic as it gets, me playing in my kitchen. Welcome! :-)
In the video, I am showing the two full experiments: For small rotations we get a low latitude, laminar, tropical Hadley circulation case. Spinning faster, we get a baroclinic instability, eddying, extra-tropical case. And as you’ll see, I didn’t know which circulation I was going to get beforehand, because I didn’t do the maths before running it. I like surprises, and luckily it worked out well!