Tag: ocean circulation


Combining a slowly rotating water tank with a temperature gradient: A thermal wind demonstration!
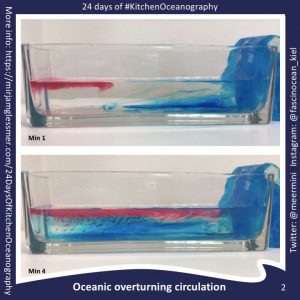
Setting up an overturning circulation in a tank is easy, and also interpreting the observations is fairly straightforward. Just by introducing cooling on one side of a rectangular tank a…

Stuff you can (and should!) observe in your kitchen: circulation in the water when boiling eggs
Now that I have introduced the new tag “kitchen oceanography: food related” to my blog, it’s time to add a couple new posts to that category. And today I have…