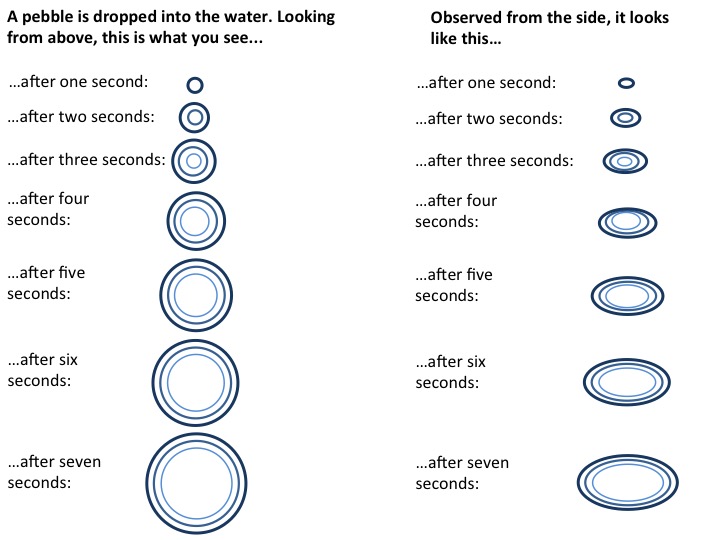
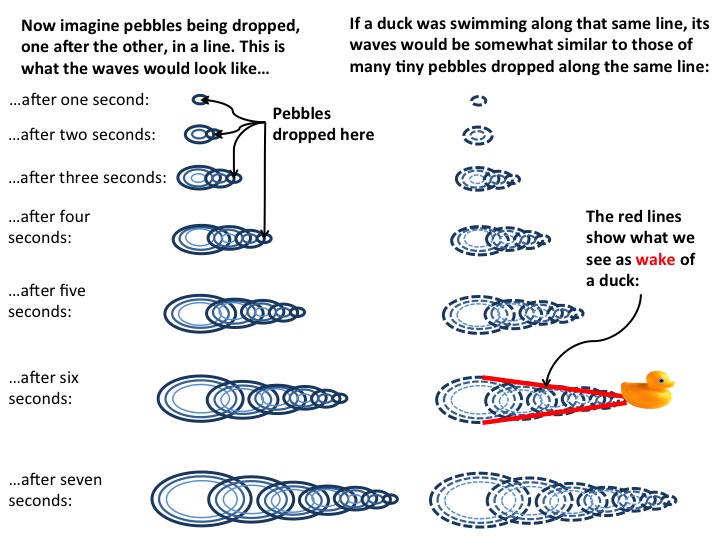
I recently went on a trip to Heligoland, Germany’s only island that is far away from the mainland (70 km in this case). It was a great trip, and I know you’ll be reading about it for some weeks to come :-)
Today, we’ll just do the touristy parts, though, and get into the oceanography later.
The trip started out super awesome. I went on a ferry and got the chance to see ships being greeted by Wilkomm Höft, the Ship Welcome Station, by dipping the Hamburg flag and playing the national anthem. I’ve seen this before, but only from land (this is a place that I love going to for birthdays, mine and other people’s). Sitting on a ship and being bid farewell this way is really touching! I loved it and got a little teary-eyed.

Being bid farewell (by dipping the Hamburg flag and playing music) at Wilkomm Höft in Wedel on the Elbe river.
The ferry itself is a high speed catamaran, which, as you might have guessed, produces an amazing wake.
Unfortunately, the ship is set up such that the rescue boats obscure the view of the wake a little. How inconsiderate ;-) Please ignore the outboard engine…
On Heligoland itself, there are the famous red cliffs, and tons of birds. Let me just show you a few:
The best-known part of Heligoland is the Lange Anna — a red rock called “long Anna”. You see it on the picture below. What I liked most about it — besides the beauty of the rock and the birds, obviously — was the wave breaker build there to protect the coast, and what it did to the waves.
Zooming in on the wave breaker’s edge, there is clearly very strong winds coming around that corner:
To be fair, it was a super windy day.
They also have a very nice beach on Heligoland, where you can see the bending of waves due to changes in topography that we talked about before.
And if you look over to the second island, you can very nicely compare and contrast the upwind and downwind coasts of islands:
See how there are lots of breaking waves (well, you probably only see the foam) on the upwind coast of the island in the back of the image, and how there are absolutely no waves in the lee of the sea wall in the foreground of that picture?
Btw, the downwind side of that same second island looks also a lot calmer as it is sheltered by the island itself:
Unfortunately, I couldn’t take a picture of the whole island at once, I really need to upgrade my camera… But can you spot the rainbow above?
Also there is some more, pretty spectacular weather to be seen:
If you go over to the other island, there are TONS of seals. Like several hundred, right on the beach! Obviously, I only have pictures of seals when there are also nice waves happening at the same time :-)
Oh, and birds.
And more seals! This one is cute, I have to admit…
Oh, and I was lucky (lucky means bold enough to ask!) enough to hitch a ride on the local research vessel, Aade. Below, they just finish a plankton trawl. We weren’t allowed out on deck because the weather was so rough…
What looks like a calm and serene morning really wasn’t one. It was super windy and wavy! Good thing I don’t get sea sick.
It turns out it’s super difficult to take pictures of waves that look as impressive as the waves are in reality. I really need to learn how to do that! But even if you don’t see the size of the waves, at least you get a different look at “lange Anna” below.
And these are white caps on the “open” sea, not waves breaking on a shore.
And for a nice comparison: The Aade (the research vessel I was on) and the catamaran that took me home later that night.
Anyway, it was a great trip!
















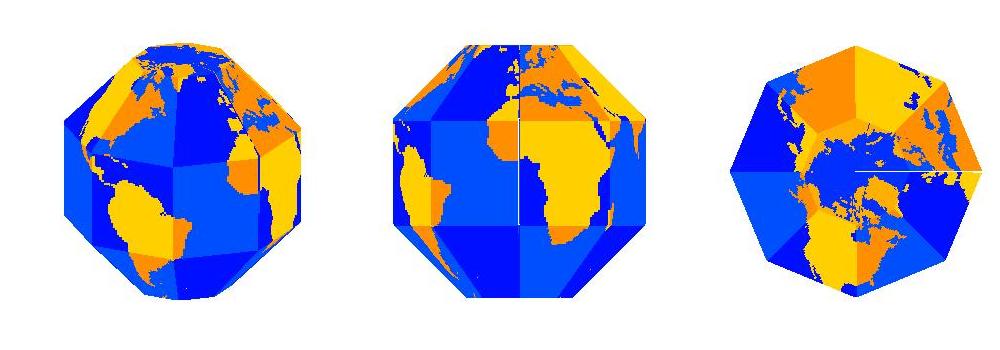


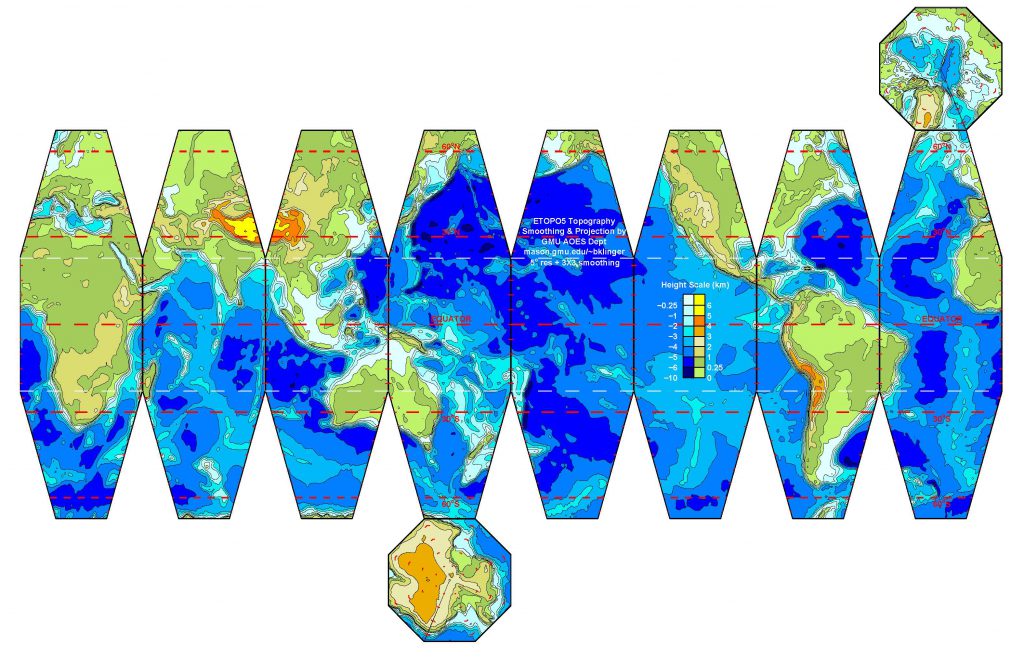
 Once a map is projected on to a flat image, like the one above, and printed out, I can create a SectorGlobe after about 20 minutes of cutting and pasting (with actual scissors and tape, not Microsoft Office products!). The sectors also have to be folded along the white lines. It is rather entertaining seeing the object snap into the correct shape as the last few edges are connected.
Once a map is projected on to a flat image, like the one above, and printed out, I can create a SectorGlobe after about 20 minutes of cutting and pasting (with actual scissors and tape, not Microsoft Office products!). The sectors also have to be folded along the white lines. It is rather entertaining seeing the object snap into the correct shape as the last few edges are connected.