Tag: circulation

Combining a slowly rotating water tank with a temperature gradient: A thermal wind demonstration!
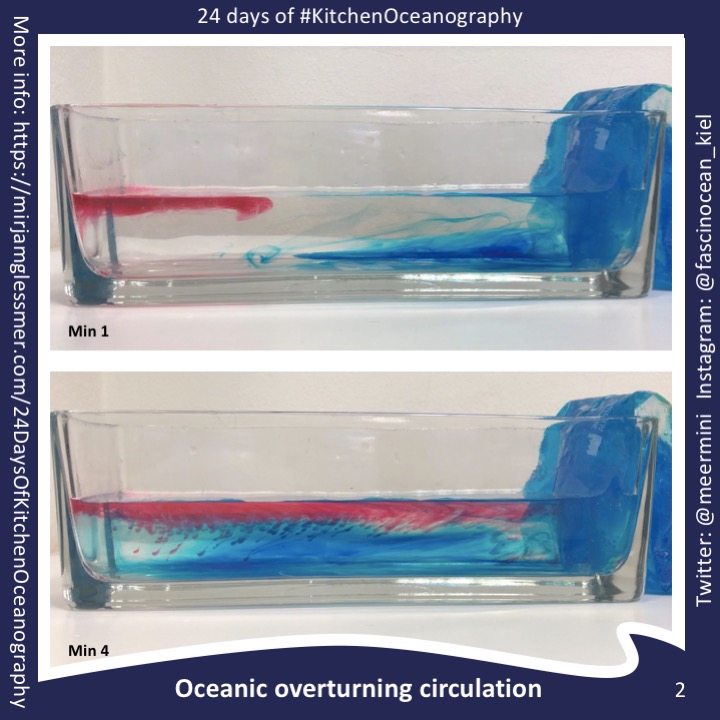
Setting up an overturning circulation in a tank is easy, and also interpreting the observations is fairly straightforward. Just by introducing cooling on one side of a rectangular tank a circulation is induced (at least for a short while until the tank fills up with a cold pool of water; see left plot of the […]
Experiment: Temperature-driven circulation
My favorite experiment. Quick and easy and very impressive way to illustrate the influence of temperature on water densities. This experiment is great if you want to talk about temperature influencing density. Although it doesn’t actually show anything different from a temperature driven overturning experiment, where circulation is determined by hot water rising and cold […]
Those foam stripes parallel to the coast — again!
I think I might be getting obsessed with those stripes parallel to the coast. We saw them as foam stripes, eel grass stripes and now today: leaf stripes! Or should it be leaves stripes instead of leaf stripes? Interestingly enough, that day there wasn’t just one stripe, but in some places there were even two. It’s […]
The importance of playing in outreach activities.
Some time ago, I wrote two blog posts on the importance of playing in outreach activities for the EGU’s blog’s “educational corner” GeoEd. Both have now been published, check them out! Here is the link on EGU’s website (here) and in case that ever stops working, it is also available on my own website (here […]
Continuity
What do you see when you look at an aquarium? When I was in Gothenburg last year for EMSEA14, one night we got to hang out at the Sjöfartsmuseet Akvariet there, and, even cooler, had the whole place to ourselves. A lot of the staff was around and happy to chat, including people who actually designed […]
Eddies in a jar
Rotating experiments in your kitchen. Do you know those Saturday mornings when you wake up and just know that you have to do oceanography experiments? I had one of those last weekend. Unfortunately, I didn’t have a rotating table at hand, but luckily most of my experiments work better than the exploding water balloon time-lapse […]
Hadley cell experiment
Cooling and rotation combined. (deutscher Text unten) I can’t believe I haven’t blogged about this experiment before now! Pierre and I have conducted it a number of times, but somehow the documentation never happened. So here we go today! Martin and I ran the experiment for our own entertainment (oh the peace and quiet in […]
Langmuir circulation, take 2
Attempt at mechanistic understanding of Langmuir circulation. After complaining about how I didn’t have mechanistic understanding of Langmuir circulation recently, and how I was too lazy to do a real literature search on it, my friend Kristin sent me a paper that might shed light on the issue. And it did! So here is what I […]
Water seeks its level.
A solution for the siphon problem of the fjord circulation experiment. After having run the fjord circulation experiments for several years in a row with several groups of students each year, Pierre and I finally figured out a good way to keep the water level in the tank constant. As you might remember from the […]
Fjord circulation
Tank experiment on a typical circulation in a fjord. Traditionally, a fjord circulation experiment has been done in GEOF130’s student practicals. Pierre and I recently met up to test-run the experiment before it will be run in this year’s course. This is the setup of the experiment: A long and narrow tank, filled with salt water, […]
Langmuir circulation
We think we observed Langmuir circulation, but we don’t understand the mechanism causing it. Recently, my friend Leela came to visit Bergen and we went on a fjord cruise to make the most of a sunny October day. We observed foam streaks on the fjord. The structures were long and persistent, and being the oceanographers […]