
Why melting sea ice does not contribute to sea level rise.
Simple experiment on why the impact of glaciers and sea ice on sea level, respectively, are not the same.
It could be so simple: An ice cube sinks into water until the mass it replaces is equal to its own mass.

The mug is as full with water as it gets. But even if I stared out of the window at the mountain and the snow until this swimming piece of ice had completely melted – the water level in the mug would not have changed.
Since the mass of said ice cube is not changing when it melts, under the assumption that the difference in volume due to the temperature difference of the melt water and the water in which the ice cube swims is negligible (reasonable assumption in most cases) that means that a swimming ice cube can’t change the water level in a cup and a swimming ice berg can’t change sea level. Things are different for glaciers or other ice that is sitting on land rather than freely swimming.

I should have thought about how I would transport the plate on which the mug with the ice is sitting back to the kitchen once the ice has melted. In other words: Yes, the mug will spill over.
This is a very easy demonstration and while it is intuitive that in the second case a mug that was completely filled with water when the ice was first added will spill over once the ice melts, the first case seems to be very difficult. Most students are not quite sure what they are expecting to see, and even if they are, they don’t really know why.
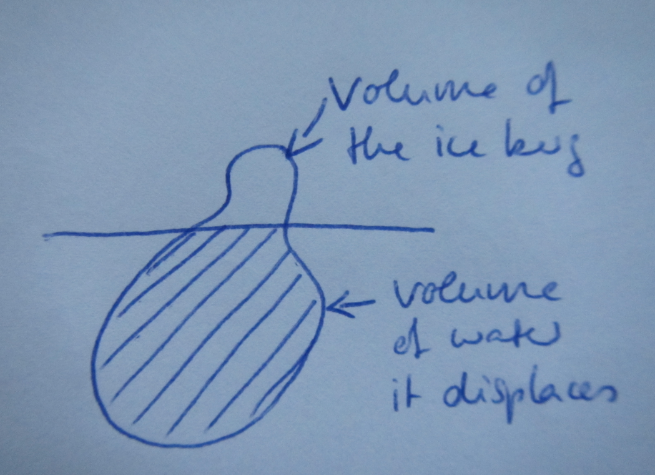
My typical drawing to explain this topic. The potato is supposed to be an ice berg floating in water.
I have always been teaching this by drawing the water level and the ice berg on the board, and then by marking the volume of the whole ice berg and the part of it that is under water, and trying to stress how the mass of the ice berg is the same as that of the water replaced by the part of the ice berg that is under water (because the molecules are more densely packed in liquid water and yada yada) — there must surely be a better way to explain this? Any ideas out there?

Why “But I don’t have anything interesting to say, I am just teaching a block course right now” is not a good excuse for not tweeting | Dr. Mirjam S. Glessmer says:
[…] that melting sea ice doesn’t contribute to sea level rise?” (Of course, she does have an experiment in her repertoire, this is just an […]