Tag: ocean circulation

Combining a slowly rotating water tank with a temperature gradient: A thermal wind demonstration!
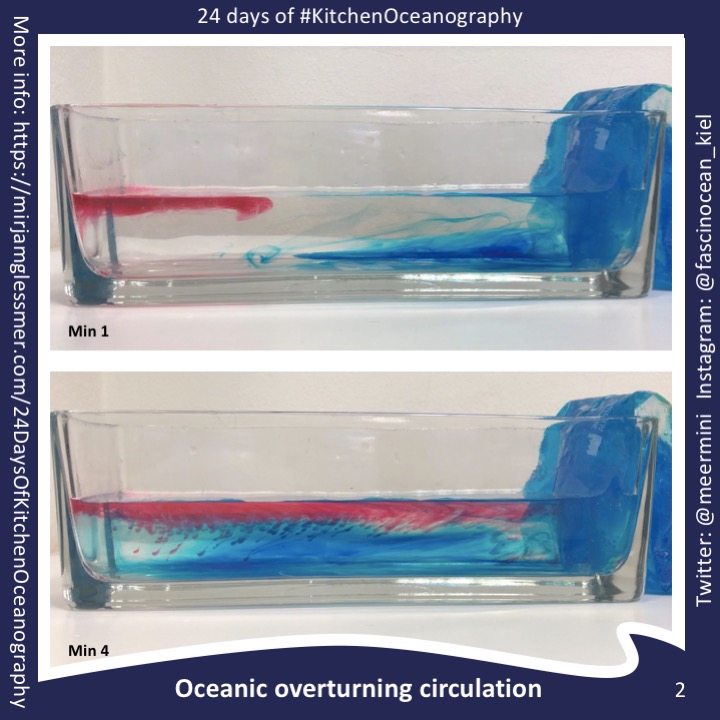
Setting up an overturning circulation in a tank is easy, and also interpreting the observations is fairly straightforward. Just by introducing cooling on one side of a rectangular tank a circulation is induced (at least for a short while until the tank fills up with a cold pool of water; see left plot of the […]

Stuff you can (and should!) observe in your kitchen: circulation in the water when boiling eggs
Now that I have introduced the new tag “kitchen oceanography: food related” to my blog, it’s time to add a couple new posts to that category. And today I have a fun post for you! But first, what does “kitchen oceanography” even mean? Kitchen oceanography /ˈkɪtʃɪn ˌəʊʃəˈnɒɡrəfi/ noun Experiments on processes related to the ocean […]