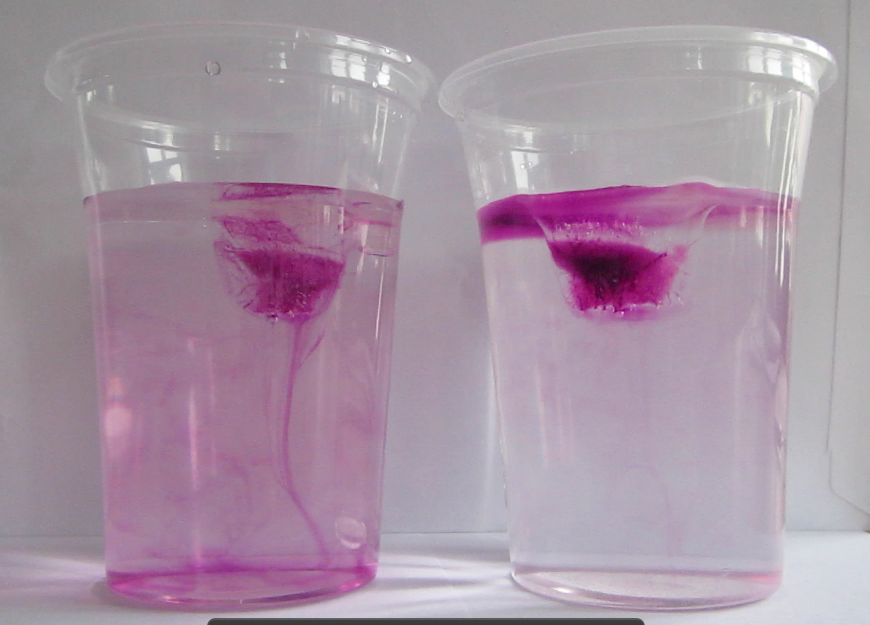
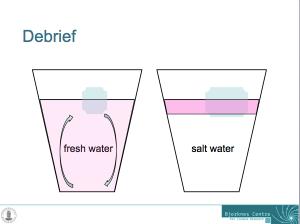
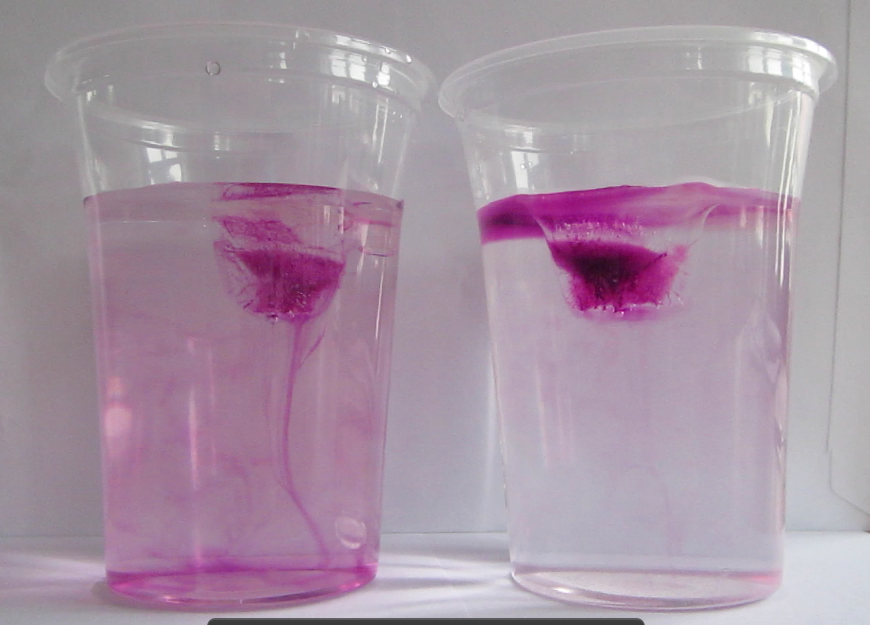
What contexts can the “ice cubes melting in fresh water and in salt water” experiment be used in?
As you might have noticed, I really like the “ice cubes melting in fresh water and in salt water” experiment. Initially, I had only three posts planned on the topic (post 1 and 2 showing different variation of the experiment and post 3 discussing different didactical approaches to the experiment), but here we are again. Since I like this experiment so much – here are suggested contexts in which to use the experiment.
1) The scientific method.
No matter what introductory class you teach, at some point you will talk about the scientific method. And what is better than talking about the scientific method? Correct, having students experience the scientific method! This experiment is really well suited for that, because you can be fairly sure that most students will come up with a hypothesis that their experiment will not support.
2) Laboratory protocols.
For courses that include a laboratory component (like mine does), at some point you will have to talk about how to document your experiments. Again, since the hypothesis will typically not be supported by the results of the experiment, this is a great example on how important it is to write down the hypothesis and how you are planning on testing it, and then noting all the observations, not only the one that are along the lines of what you suspected. Also recording the little errors that occur along the way (“someone swapped the cups with the ice cubes, so we are not sure any more which one is which”) is very important, and if you have a class doing this experiment, you can be sure that at some point someone will make a mistake, not write it down and then be very confused afterwards. Great teaching and learning opportunity!
3) Different teaching methods.
If you are teaching about didactical models, this experiment is very well suited for this, too (see my post 3 on the topic and the Lawrence Hall of Science resource). Just have different people work on the experiment using the different methods and then discuss what and especially how people learned using those methods. The Lawrence Hall of Science resource mentions a fourth method (and I didn’t want to give the impression that I am recommending it, therefore I omitted it in my post 3) – the “read and answer” method, where students read about density, stratification and density-driven circulation and then answer questions like “what is density?” or “what is thermohaline circulation”. Again, not recommended for your oceanography class, but adding this option might be very relevant if you are teaching students or educators how to (not) teach.
4) Oceanography and climate
Yes, this is probably the main reason why you are doing this experiment in class. Now you can talk about salt in the ocean. About density-driven currents (and are there other things that drive currents apart from density differences?). About the importance of ocean currents, heat transport, the global overturning circulation, fresh water and many more.
Can you think of more contexts for this great experiment? Let me know! (Depending on your browser, you can comment on this post in the “leave a reply” box below or, if you don’t see that box, by clicking the speech bubble next to the title of the post.)