
Melting ice cubes & thermal imaging camera
I haven’t talked about my favourite experiment in a long time (before using it last week in the MeerKlima congress and suddenly talking about it all the time again), because I felt like I had said everything there is to say (see a pretty comprehensive review here) BUT! a while back my colleagues started playing with a thermal imaging camera and that gave me so many new ideas! :-)
I showed you this picture yesterday already:

Here we see ice cubes melting in fresh water and salt water (and my very fancy experimental setup. But I am pretty proud of my thermal insulation!). Do you know which cup contains which?
Here are some more pics: The ice cubes before being dropped into the cups. Clearly dark purple is cold and yellow/white is warm (see my fingers?)

After a while (5ish minutes), the cold meltwater has filled up the bottom of the freshwater cup while floating on top of the salt water cup:

Looking in from the top, we see that the ice cube in salt water hasn’t melted yet, but that the other one is gone completely and all the cold water has sunk to the bottom of the beaker.
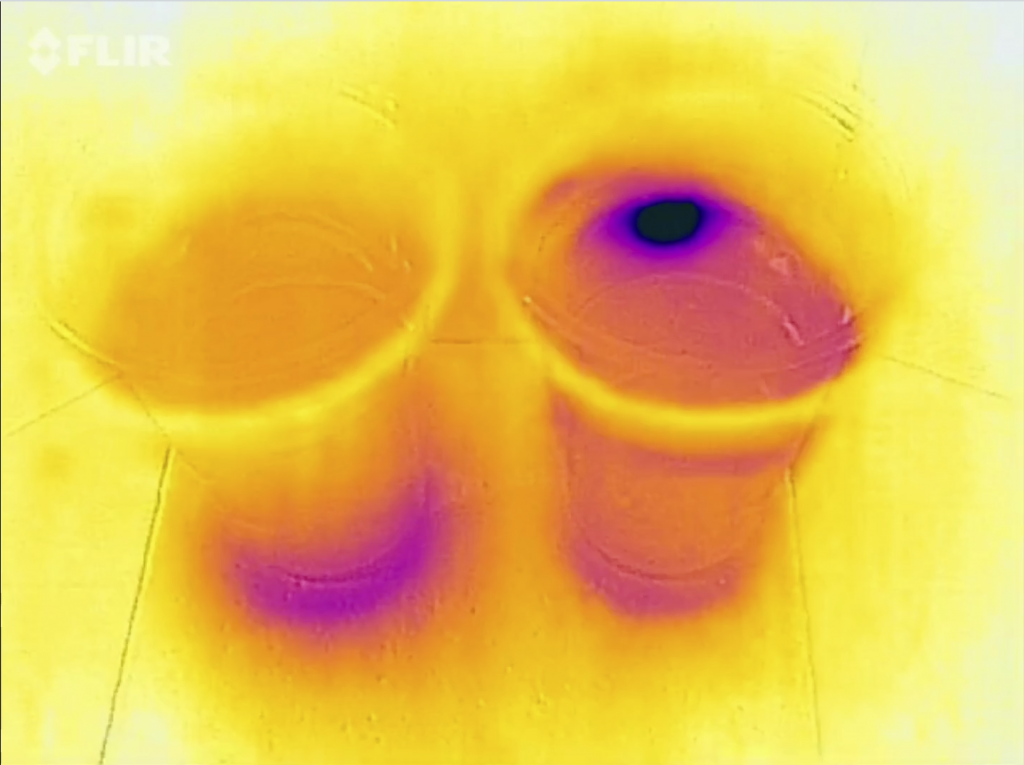
When you check out the movie at the bottom of this post, you will notice that this experiment doesn’t work quite as well as I had hoped: In the saltwater cup, the ice cube floats against the wall of the cup and for quite some time it looks like there is a plume of cold water sinking in the salt water. I’m not quite sure what’s going on there. If it’s showing up like that because the cup is such a good thermal conductor, then why is the “plume” directional and not spreading in all directions? If there really is a plume, then how did it get there? It shouldn’t be! So many questions!
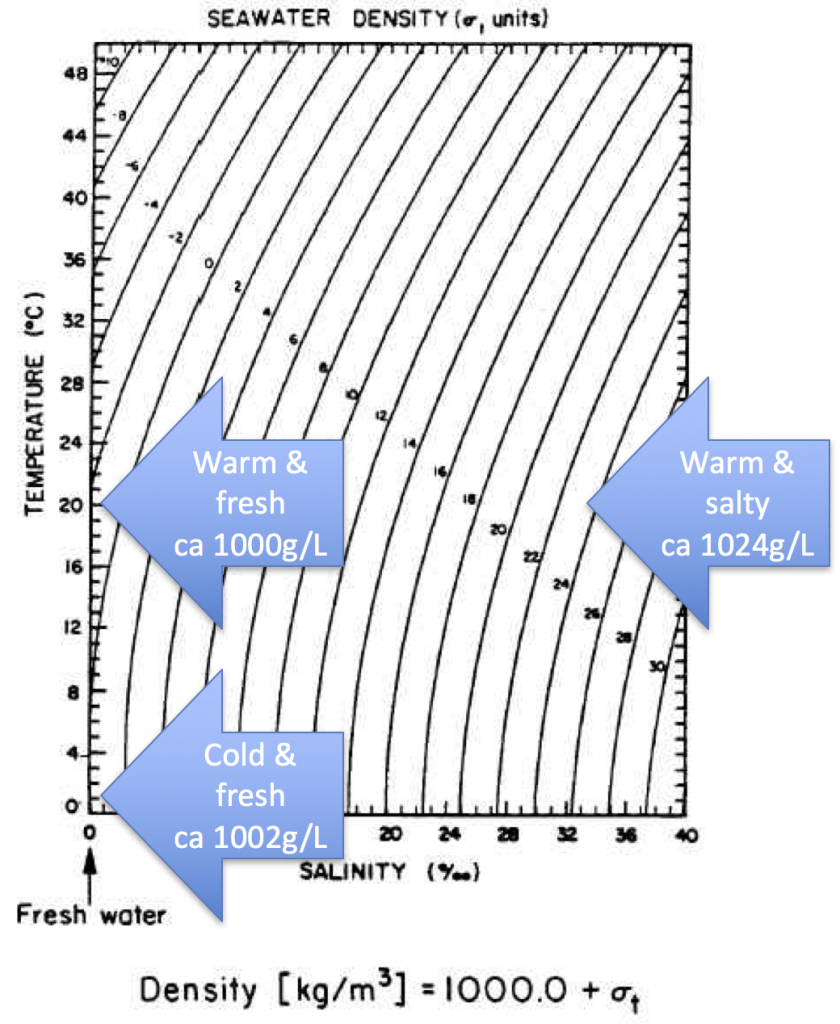
There really can’t be a plume of cold melt water in the salt water cup. For my workshop last week I made the plot below (which, btw, I don’t think anyone understood. Note to myself: Explain better or get rid of it!). So unless the plume is cold salt water, there is no way anything would sink in the salt water cup.
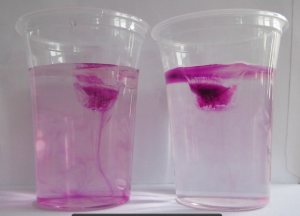
So maybe we are cooling the salt water around the ice cube which then sinks and shows up because it is close to the wall of the cup? We can’t look “into” the cup with a thermal imaging camera, we can only see the surface of the cup (See, Joke? Maybe it is useful after all to learn all that stuff in theoretical oceanography ;-)). That’s also why we don’t see a plume of cold melt water in the freshwater case like we see when we have dyed ice cubes and see the melt water plume, like below:
Anyway. Here is the video, in which you sometimes see my finger, pushing the ice cube away from the beaker’s wall to finally get to a state that looks like what I wanted to show you above:
Melting ice cubes experiment — observing the finer details – Mirjam S. Glessmer says:
[…] This, not surprisingly, looks very similar to what a thermal imaging camera sees when observing the experiment (as described in this post). […]