I’m very excited to announce that I, together with Christian Seifert, have been awarded a Tandem Fellowship by the Stifterverband für die Deutsche Wissenschaft. Christian, among other things, teaches undergraduate mathematics for engineers, and together we have developed a concept to improve instruction, which we now get support to implement.
Tag Archives: teaching
What does the awkward silence mean?
I really want to recommend a blog post by Paul T. Corrigan that I recently read on “Teaching and Learning in Higher Ed”: When students don’t answer a question, what does the awkward silence mean?
We’ve all been there: We’ve asked a question and nobody replied. Worse, even, they avoid our eyes. What can we do? Check out the post for a surprisingly simple idea!
Guest post: Estimating salinity as a homework assignment
Today I am super excited to share a guest post that my awesome friend Joke Lübbecke wrote for us. Joke is a professor in physical oceanography in Kiel, and we like to chat about teaching occasionally. She has great ideas for exciting tasks for students to do and I bet they learn a lot from her. Here is what she writes (and the photos in this post are the original photos that her students kindly agreed to let us use on this blog. Thanks very much!):
Estimating salinity as a homework assignment
When I gave the second-year oceanography students in my class bottles of salt water and – without any further instructions – asked them to find out what the salinity was, I wasn’t really sure what to expect. Would they just take a sip and guess 35? Would they all use the same approach? So when they handed in their solutions in the following week I was very happy to see how creative they had been and how many different things they had tried to get to an answer. For example, they had
- Evaporated the water and weighted the dry salt
- Used differences in buoyancy between salt and fresh water
- Measured the electric resistance of the sample, then tried to mix a solution with the same resistance by adding more and more (defined quantities of) salt to a fresh water sample
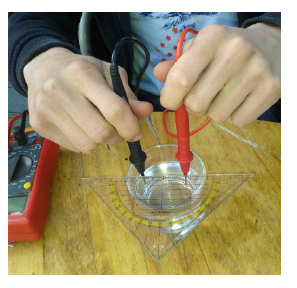
Measuring salinity by measuring the resistance of the sample and reproducing a sample with known salinity and the same resistance
or simply
- Tasted the sample and compared to water samples with known salinities :-)
The numbers they came up with were as diverse as their approaches so this was also a nice demonstration of the difficulties to accurately measure salinity.
(And of course the salinity of the water sample they got was about 35, but who cares? – the journey is the reward!)

Facilitating student group work
-> the crisis clinic
Another measure that the authors suggest is to occasionally run “crisis clinics”, i.e. short sessions on problematic behaviors, like for example hitchhiking, and putting students together to brainstorm how to deal with those issues. Collecting ideas serves two purposes: To show hitchhikers how frustrated the rest of the group might get with their behavior, and also to equip everybody with the strategies to deal with that kind of behavior.
But it is also important to point out to students that if they continue putting a hitchhiker’s name on the group assignment, they can’t complain later.
Puuuuh. The authors continue on, talking about peer grading and going through a long list of FAQs, but I think for today I’ve written enough. But check out the paper, there is so much more in there than I could talk about here!
—
Barbara Oakley, Rebecca Brent, Richard M. Felder, & Imad Elhajj (2004). Turning Student Groups into Effective Teams New Forums Press, Inc., P. O. Box 876, Stillwater, OK
Rogue waves in a bath tub
Trying to create rogue waves in the bath tub of the infamous “red house”.
As a part of their projects, students in the CMM31 in Isafjördur course had to conduct an experiment, document and interpret it. One of the students, Silvia, chose to create rogue waves in the bath tub of the “red house”, one of the student houses, and I was invited to participate and eat delicious cupcakes.
Since rogue waves can have devastating effects on ships they encounter, clearly we had to have a ship. None were to be found, so we had to make our own.
Since most studies of rogue waves in wave tanks had a hard time actually producing the waves (and a bathtub might not be the most ideal setup) we did not have high hopes that our experiment would be successful. And we did not manage to produce rogue waves in the strict sense – but we managed to avoid major spillage of the tub and still sink a couple of the paper boats, so at least we were getting some results.
Great to see students do experiments on a Sunday afternoon!
How sound is refracted towards the regions of minimum speed.
Students acting out the process of sound being refracted towards the region of minimum speed.
We’ve been talking about refraction lately. Waves get bent in the direction of lower velocity. This holds for light and sound and even ocean waves. However, students find it conceptually difficult to understand why waves are being bent towards lower rather than higher speeds, so I came up with this very simple demonstration.
Students, arms joint, are acting as a wave crest. Students on the one side of the student chain are told to move very slowly, students on the other side are asked to move quickly towards the instructor. Everybody takes care to not hurt anybody, so if tension builds up in the chain, everybody has to react to reduce the tension. What happens is that the “wave crest” of students changes direction towards the side of the slowest motion.
Easy visualization and – since it involved students getting up, joining arms and doing something – also very memorable. Win – win!
Another easy example: When you are sliding on an icy road and your foot gets caught in grass or gravel or something on one side (== region of lower velocity), you start skidding towards the side with the obstacle, not towards the middle of the icy road.
Examinations via Skype.
My experience with an examination via Skype.
In 2012, I taught two lectures via Skype at the University Centre of the Westfjords, while actually physically sitting in Norway. That experience is described in this post. When writing that post, I remembered that I also have experience in doing examinations via Skype. Except that experience was as a student, not as a teacher. In 2011, I defended a Master’s thesis at the University of Hamburg while, again, being physically located in Norway. How did that work out?
Defending a thesis via Skype is not that uncommon these days and actually a very easy, cheap and environmentally friendly way of defending when you no longer live in the place where you studied (or when you cannot travel there for other reasons). The way it worked in my case was that I had two opponents on the call, and since we were all to cheap for the upgrade, we could only hear each other and did not have a video connection. Which made it less stressful for me – when I am video-skyping, I tend to focus on my own video way too much, and thinking about how weird my hair looks or how I should sit in a specific position to block something behind me that would otherwise be visible. This tends to take away brain power from the topic I should be focussing on. Since I knew both their voices, there was also not an issue with knowing who was speaking at any given time (if you are ever on a call/skype with a group of people and there is even one person who doesn’t know everybody else really well: Please make sure to always announce who you are when you start speaking!).
I had to give a presentation, which I did by sending them the slides in advance and asking them to look at specific slides while I was talking about them. Thanks to my friend Nadine who let me borrow her apartment, I had a fast internet connection and privacy. What more do you need?
The only stressful time was waiting for them to call back after the exam when they were discussing my grade, but I guess that is a really stressful time no matter the setting.
So yes – examinations via Skype are actually a good option! No bad experiences here.
Hydrothermal springs
Hydrothermal springs that you can visit without a deep-sea submersible.
When teaching about hydrothermal springs, I usually use a video a friend of mine took of hydrothermal vents on the mid-Atlantic ridge on the WHOI submersible Alvin. But being on Iceland now, there is much better material available which students can even go and experience themselves.
I am too chicken to take my camera under water in the Blue Lagoon to film the hot springs, but there are other hot springs all over Iceland that are less scary, for example this one that my friend Astrid found in the middle of a meadow.

View from the top into the hot spring – do you see the bubbles breaking the surface?
And here I even dared take my camera under water.

View of the hot spring under water – that’s where the bubbles come from!
Granted, this is not quite as impressive as a black smoker or the Blue Lagoon. But the water in the whole little lake was warmer than about 40 degrees Celsius, and the hot spring is sitting randomly in a field. That’s hand-on geothermal heating for you!
On drawing on the board by hand in real time
Drawing by hand on the board in real time rather than projecting a finished schematic?
It is funny. During my undergrad, LCD projectors were just starting to arrive at the university. Many of the classes I attended during my first years used overhead projectors and hand-written slides, or sometimes printed slides if someone wanted to show really fancy things like figures from a paper. Occasionally people would draw or write on the slides during class, and every room that I have ever been taught in during that time did have several blackboards that were used quite frequently.
These days, however, things are differently. At my mom’s school, many classrooms don’t even have blackboards (or whiteboards) any more, but instead they have a fancy screen that they can show things on and draw on (with a limited number of colors, I think 3?). Many rooms at universities are similarly not equipped with boards any more, and most lectures that I have either seen or heard people talk about over the last couple of years exclusively use LCD projectors that people hook up to their personal laptops.
On the one hand, that is a great development – it is so much easier to show all kinds of different graphics and also to find and display information on the internet in real time. On the other hand, though, it has become much more difficult to talk students through graphics slowly enough that they can draw with you as you are talking and at the same time understand what they are drawing.
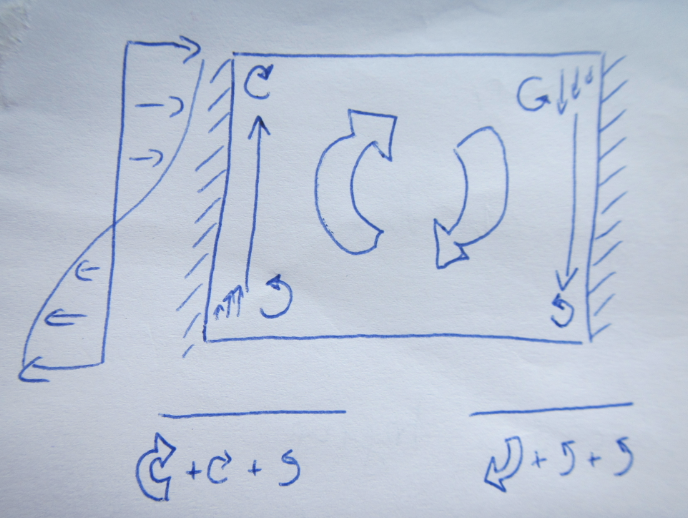
Sketch of the mechanisms causing westward intensification of subtropical gyres – here the “before” stage where the symmetrical gyre would spin up since the wind is inputting more vorticity that is being taken out by other mechanisms.
The other day, I was teaching about westward intensification in subtropical gyres. For that, I wanted to use the schematics above and below, showing how vorticity input from the wind is balanced by change in vorticity through change in latitude as well as through friction with the boundary. I had that schematic in my powerpoint presentation, even broken down into small pieces that would be added sequentially, but at last minute decided to draw it on the whiteboard instead.

Sketch of the mechanisms causing westward intensification of subtropical gyres – here the “after” stage – the vorticity input by wind is balanced by energy lost through friction with the western boundary in an asymmetrical gyre. Voila -your western boundary current!
And I am convinced that that was a good decision. Firstly, drawing helped me mention every detail of the schematic, since I was talking about what I was drawing while drawing it. When just clicking through slides it happens much more easily that things get forgotten or skipped. Secondly, since I had to draw and talk at the same time, the figure only appeared slowly enough on the board that the students could follow every step and copy the drawing at the same time. And lastly, the students saw that it is actually possible to draw the whole schematic from memory, and not just by having learned it by heart, but by telling the story and drawing what I was talking about.
Does that mean that I will draw every schematic I use in class? Certainly not. But what it does mean is that I found it helpful to remember how useful it is to draw occasionally, especially to demonstrate how I want students to be able to talk about content: By constructing a picture from scratch, slowly building and adding on to it, until the whole theory is completed.
Why do we get an Ekman spiral?
Visualizing an Ekman spiral using a deck of cards.
To state this right upfront: this post will not explain why the surface layer is moving at a 45 degree angle to the wind direction, and if anyone has a great idea for a simple demo for that please let me know! It will also not explain why the layers are turning further and further the deeper down you go. But what I am trying to do today is give an intuitive understanding for why all the theoretical layers in the water column turn in response to the surface layer and hence why an Ekman spiral develops if we accept that the surface layer is turning relative to the wind direction.
You will need a deck of cards. Bonus points if they are “salmon fly” cards like mine (seriously – who could walk past a deck of cards with salmon flies on them? Plus I needed a deck of cards because I was already in Iceland when I realized I wanted to show this demo).
All you do now is put the stack in front of you. Put your hand on the top card, twist gently while applying a little bit of pressure. Voila – your Ekman spiral develops! It is turning the wrong way round, but the main point is that the twist is being transferred downwards from layer to layer and not only the top layer twisting while the other layers stay motionless.
And because people seem to always like movies: