Because I am getting sick of stratifications not working out the way I planned them.
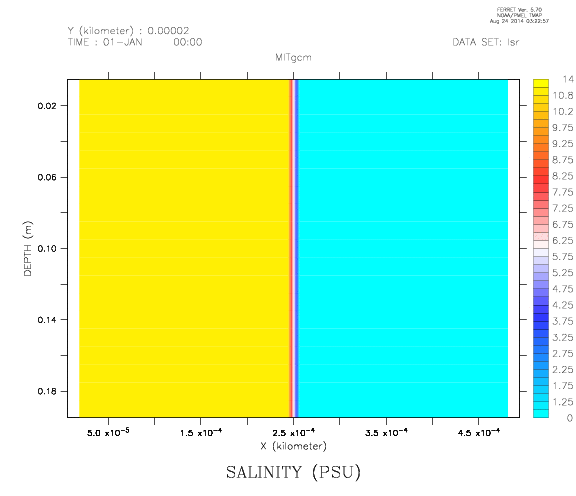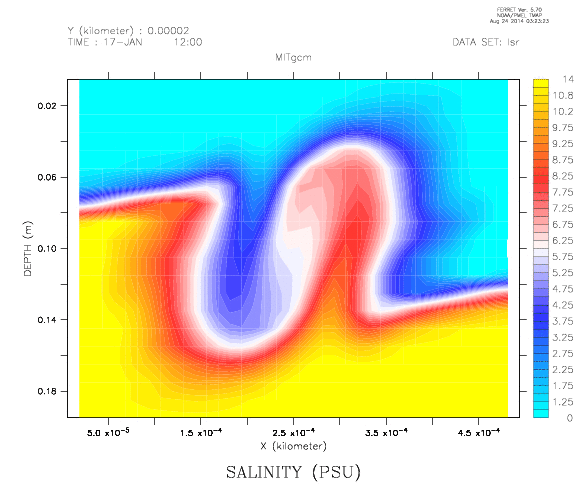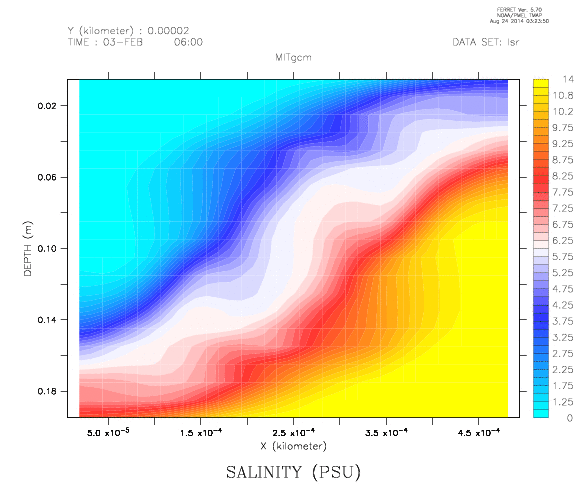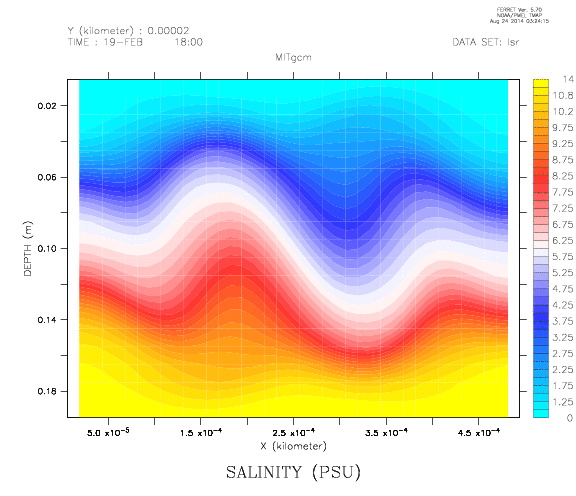
Creating stratifications, especially continuous stratifications, is a pain. Since I wanted a nice stratification for an experiment recently, I finally decided to do a literature search on how the professionals create their stratifications. And the one method that was mentioned over and over again was the double bucket method, which I will present to you today.
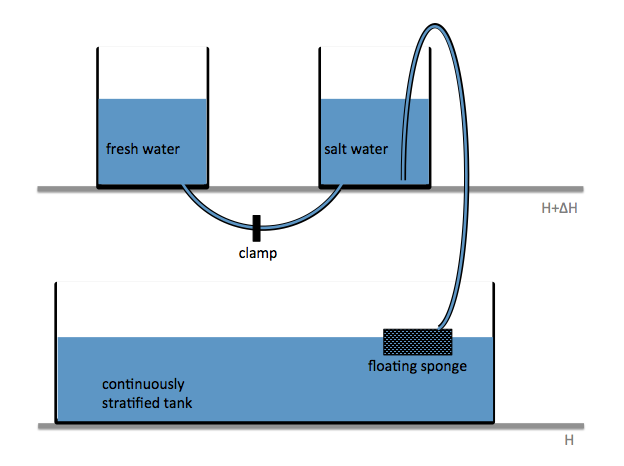
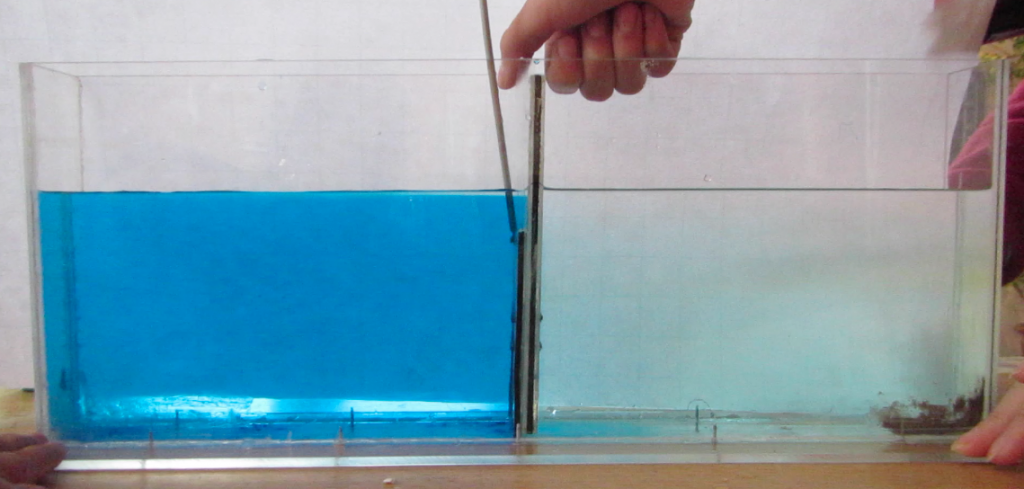
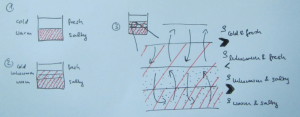
Two reservoirs are placed at a higher level than the tank to be filled, and connected with a U-tube which is initially closed with a clamp. Both reservoirs are filled with fresh water. To one of the buckets, salt is added to achieve the highest desired salinity in the stratification we are aiming for. From this bucket, a pump pumps water down into the tank to be filled (or, for the low-tech version: use air pressure and a bubble-free hose to drive water down into the tank as shown in the figure above!); the lower end of the hose rests on a sponge that will float on the water in the tank. When the pump is switched on (or alternatively, the bubble-free hose from the reservoir to the tank opened), the clamp is removed from the U-tube. So for every unit of salt water leaving the salty reservoir through the hose, half a unit of fresh water flows in to keep the water levels in both reservoirs the same height. Thus the salt water is, little by little, mixed with fresh water, so the water flowing out into the tank gets gradually fresher. If all goes well, this results in a continuous salinity stratification.
Things that might go wrong include, but are not limited to,
- freshwater not mixing well in the saline reservoir, hence the salinity in that reservoir not changing continuously. To avoid that, stir.
- bubbles in the U-tube (especially if the U-tube is run over the top edges of the reservoirs which is a lot more feasible than drilling holes into the reservoirs) messing up the flow. It is important to make sure there is no air in the tube connecting the two reservoirs!
- water shooting out of the hose and off the floating sponge to mess up the stratification in the tank. Avoid this by lowering the flow rate if you can adjust your pump, or by floating a larger sponge.
P.S.: For more practical tips for tank experiments, check out the post “water seeks its level” in which I describe how to keep the water level in a tank constant despite having an inflow to the tank.