An example of one topic at different levels of difficulty.
Designing exercises at just the right level of difficulty is a pretty difficult task. On the one hand, we would like students to do a lot of thinking themselves, and sometimes even choose the methods they use to solve the questions. On the other hand, we often want them to choose the right methods, and we want to give them enough guidance to be able to actually come to a good answer in the end.
For a project I am currently involved in, I recently drew up a sketch of how a specific task could be solved at different levels of difficulty.
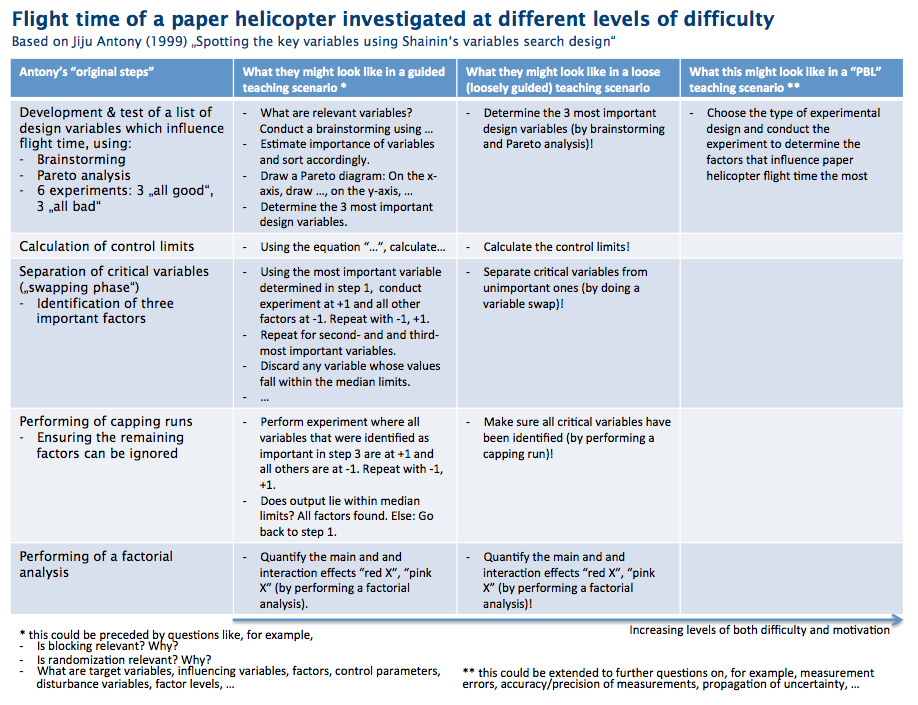
The topic this exercise is on “spotting the key variables using Shainin’s variables search design”, and my sketch is based on Antony’s (1999) paper. In a nutshell, the idea is that paper helicopters (maple-seed style, see image below) have many variables that influence their flight time (for example wing length, body width, number of paper clips on them, …) and a specific method (“Shainin’s variables search design”) is used to determine which variables are the most important ones.
In the image below, you’ll find the original steps from the Antony (1999) paper in the left column. In the second column, these steps are recreated in a very closely-guided exercise. In the third column, the teaching scenario becomes less strict, (and even less strict if you omit the part in the brackets), and in the right column the whole task is designed as a problem-based scenario.
Clearly, difficulty increases from left to right. Typically, though, motivation of students tasked with similar exercises also increases from left to right.
So which of these scenarios should we choose, and why?
Of course, there is not one clear answer. It depends on the learning outcomes (classified, for example, by Bloom or in the SOLO framework) you have decided on for your course.
If you choose one of the options further to the left, you are providing a good structure for students to work in. It is very clear what steps they are to take in which order, and what answer is expected of them. They will know whether they are fulfilling your expectations at all times.
The further towards the right you choose your approach, the more is expected from the students. Now they will need to decide themselves which methods to use, what steps to take, whether what they have done is enough to answer the question conclusively. Having the freedom to choose things is motivating for students, however only as long as the task is still solvable. You might need to provide more guidance occasionally or point out different ways they could take to come to the next step.
The reason I am writing this post is that I often see a disconnect between the standards instructors claim to have and the kind of exercises they let their students do*. If one of your learning outcomes is that students be able to select appropriate methods to solve a problem, then choosing the leftmost option is not giving your students the chance to develop that skill, because you are making all the choices for them. You could, of course, still include questions at each junction, firstly pointing out that there IS a junction (which might not be obvious to students who might be following the instructions cook-book style), and secondly asking for alternative choices to the one you made when designing the exercise, or for arguments for/against that choice. But what I see is that instructors have students do exercises similarly to the one in the left column, probably even have them write exams in that style, yet expect them to be able to write master’s theses where they are to choose methods themselves. This post is my attempt to explain why that probably won’t work.
—
* if you recognize the picture above because we recently talked about it during a consultation, and are now wondering whether I’m talking about you – no, I’m not! :-)