At the end of last year, I did a poll on Twitter, asking what people would like to see more of in 2021: Kitchen oceanography, wave watching, teaching & scicomm tips, and other things. And 2/3rds of the respondents said they wanted more kitchen oceanography!
So obviously my strategy was to do a photo shooting and prepare … Instagram posts (did I mention I asked that question on Twitter? Yeah. Don’t ask me about the logic behind that). Anyway, below is the beginning of that series (which, on Instagram, is not posted consecutively, in case you are wondering about how often people want to see me grinning at the exact same experiment…). Enjoy!
![[photo & text] Mirjam @meermini cheering with a glass filled with clear water, with a green ice cube floating in its green melt water on top of the clear layer. In the foreground a second glass with a green ice cube in it, this time floating in completely green water. Text on the photo says "Cheers to the new year" and "#KitchenOceanography"](https://mirjamglessmer.com/wp-content/uploads/2021/01/1.png)
So people tell me they want to see more #KitchenOceanography. Get ready for 2021, I come prepared! Carrying a non-alcoholic experiment with me (doesn’t look like it, does it?). Can you tell which of the glasses contains salt water and which fresh water? Both were at room temperature before I put in the (now mostly melted) ice cubes… Happy New Year! May your 2021 be full of curiosity and new experiences!
![[Photo and text] Mirjam @meermini pointing at a glass in which a green melt water layer is spreading from a green ice cube over clear water. The text says "Observation. Discovering oceanography everywhere. #KitchenOceanography"](https://mirjamglessmer.com/wp-content/uploads/2021/01/4.png)
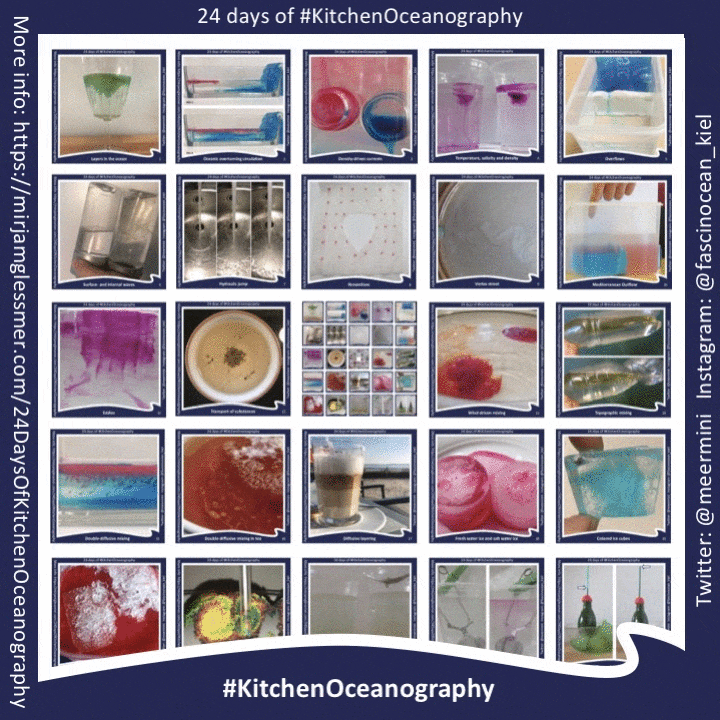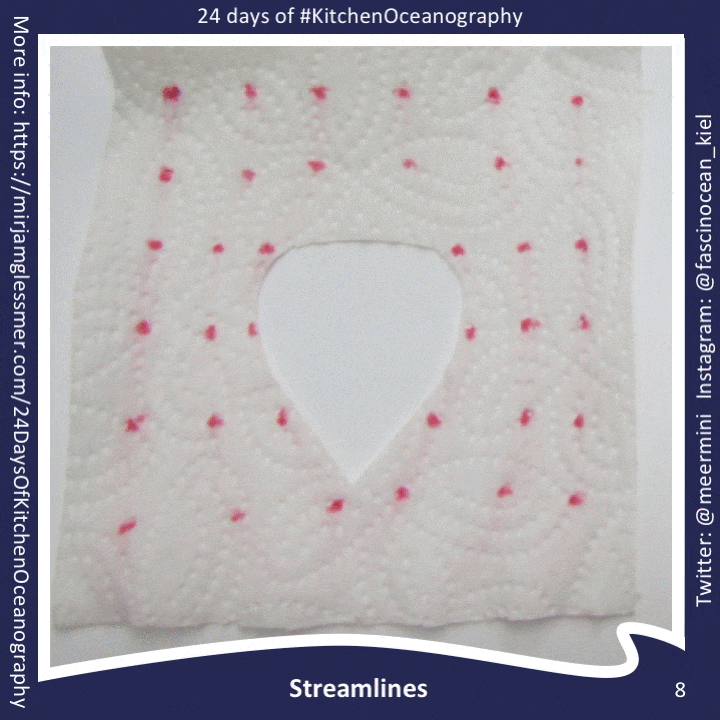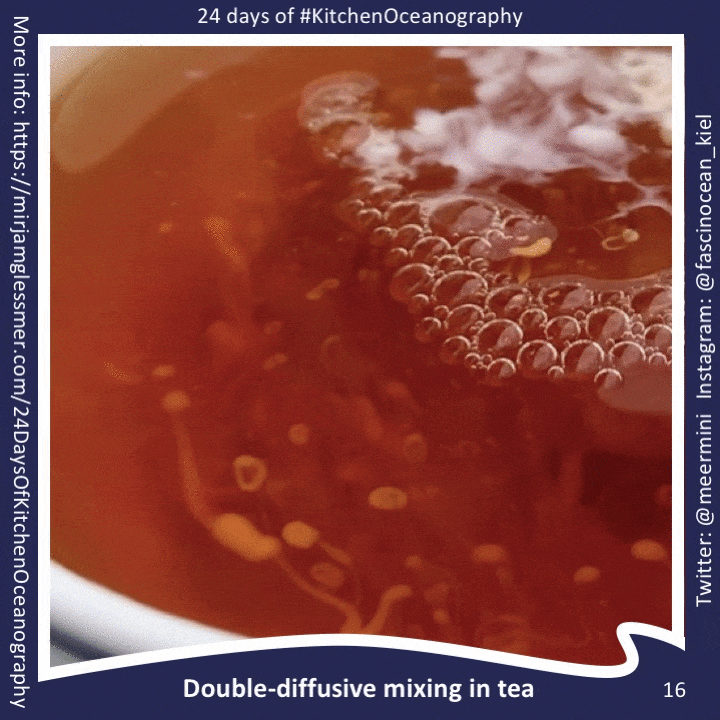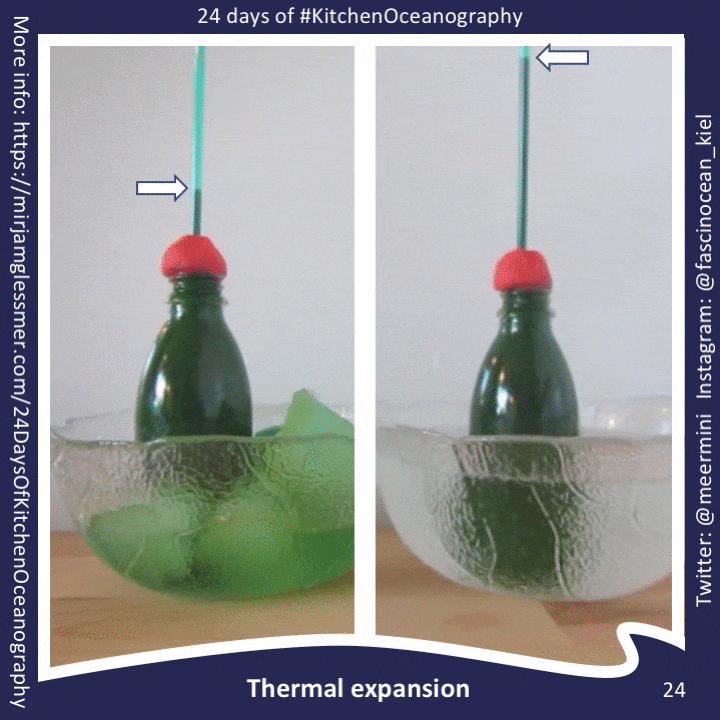
What do I love so much about #KitchenOceanography? Discovering oceanography EVERYWHERE. . When people think of oceanography, they think of endless oceans, weeks and weeks at sea on research ships, something that feels remote and unconnected to their everyday lives. But for me, it is anything but! And #KitchenOceanography is a great tool to bring the ocean and a normal everyday life closer together, both for myself and others. The concept of #KitchenOceanography is simple: you use what you find at home to simulate oceanic processes. Usually this involves some kind of “tank” (anything from a tupper ware container to the wine glass as in the picture), obviously water (usually varying temperature or salinity to change density), food dyes (or anything that can safely be used in food storage containers and that can act as coloring, e.g. dark red fruit juice, black coffee, …). And then you put it together, observe, and relate the physics happening in your kitchen to the things that happen on much larger scales in the ocean. Fun! #KitchenOceanography works really well as a fun activity at home, but is also a great tool in teaching, both in-person and remote. Over the next couple of posts I’ll tell you how and why to use it, and give you plenty of ideas for #KitchenOceanography experiments, so please check back!
![[Photo & text] Photo of Mirjam, grinning stupidly at the camera while holding green ice cubes over two glasses full of water. Text in the photo says "Excitement. Right before the experiment. #KitchenOceanography](https://mirjamglessmer.com/wp-content/uploads/2021/01/3.png)
It might not be immediately obvious to you why I am grinning stupidly at the camera in the picture above, while holding green ice cubes over two glasses full of water, so let me explain. I am about to drop the two ice cubes into the two glasses of water. But those two glasses are not filled with the same stuff. Even though it’s water at room temperature in both, one is fresh water and the other one is salt water at approximately a typical oceanic salinity (e.g. 35g of salt per liter water, or 7 tea spoons per liter). When the ice cubes are dropped into the water, they’ll both melt (in fact, they have already started, which is why I had green finger tips for days after this picture was taken). But they won’t melt in the same way. I’ve done this experiment dozens of times, alone or with people from preschool age to professional oceanographers, so I know what will happen. But what I don’t know is what EXACTLY it will look like, and what I might discover for the first time, or see more clearly than before. Even though the experiments are simple, there is ALWAYS something new to discover, because even such a simple system is still chaotic. Plumes of melt water will never look exactly the same, nor will the condensation on the glass. Discovering all those small featuers and contemplating the physics behind them makes me happy, and it’s easy to engage most people once they get over the “you are looking at two ice cubes and two plastic cups!?” threshold and actually start observing, questioning, and trying to explain, which is why #KitchenOceanography is such a great tool in teaching & outreach!

Eliciting! . #KitchenOceanography is a great tool in teaching and outreach of ocean and climate topics, because we are using a simple system — one that people think should be easy enough to intuitively understand. But this is often not the case for many reasons, one being that many people have “misconceptions” about physical processes: ideas that they formed and that worked well to explain their observations until now, but that aren’t correct and that will break down in the context of the physics we are trying to teach. . But in order for those misconceptions to be changed into correct understanding of physics, they first need to be brought to light and be made conscious. . For example by asking: In front of me you see two glasses, one filled with salt water, the other with fresh water, both at room temperature. If I drop the ice cubes in, which one will melt faster, the one in fresh water or the one in salt water? . At this point, it is not important that students give the correct answer, but that they articulate their beliefs. And what happens next? Look out for my upcoming “confront” post!

Confronting! In my previous “eliciting” post, I talked about the importance of realizing WHAT it is that we believe about how the world works. But what if our beliefs are wrong? Then, #KitchenOceanography is a great tool to confront a prediction of what SHOULD happen with what actually DOES happen. It’s surprisingly difficult to observe something that is not what we expect to observe! But when we manage to make observations that contradict what we expected to see, we come to the “confronting” step: Realising that there is a conflict between our interpretations of the world and how the world really works. So what now? Look out for my upcoming “resolving” post!

Resolving! . In previous posts, we have eliceted a misconception by asking what we believe would happen in an experiment and making predictions about the outcomes. We then confronted our prediction with an actual observation. . Now we need to somehow resolve the cognitive dissonance — what we thought should happen did not happen — and build new, correct ideas about physics into our belief system. This happens best by explaining how things fit together, either to others or to ourselves (I think the main reason I like blogging and social media is because it forces me to explain things to myself, thus helping me to understand them better!). . So this is where we talk through what we observed, what did happen, how we can explain it, what might have happened if the boundary conditions had been different. Another thing that’s great about #KitchenOceanography is that in many cases, it is very easy to test what happens when you change the boundary conditions: You CAN force the ice cubes to the bottom of the glasses and observe what happens, you CAN add salt to the water before making the ice cubes, you CAN change the water’s temperature. And then observe what happens, and see if it fits with what we expected to see. . Of course, once we get playing with #KitchenOceanography, we easily get stuck with it, changing one thing, then the next. So if you use this in teaching, be aware that it will — and should! — take a lot longer than just running an experiment once, and then moving on. But, since the experiments are so easily done with household items, students can always continue discovering outside of class, too; no fancy lab needed! Perfect! :)












![[photo] Coffee cup in which two counter-rotating eddies form because Mirjam @meermini is blowing across the cup. The eddies become visible because the circulation disrupts a surfsce film caused by cream in the coffee](https://mirjamglessmer.com/wp-content/uploads/2021/01/2.png)







![[photo & text] Mirjam @meermini cheering with a glass filled with clear water, with a green ice cube floating in its green melt water on top of the clear layer. In the foreground a second glass with a green ice cube in it, this time floating in completely green water. Text on the photo says "Cheers to the new year" and "#KitchenOceanography"](https://mirjamglessmer.com/wp-content/uploads/2021/01/1.png)
![[Photo and text] Mirjam @meermini pointing at a glass in which a green melt water layer is spreading from a green ice cube over clear water. The text says "Observation. Discovering oceanography everywhere. #KitchenOceanography"](https://mirjamglessmer.com/wp-content/uploads/2021/01/4.png)
![[Photo & text] Photo of Mirjam, grinning stupidly at the camera while holding green ice cubes over two glasses full of water. Text in the photo says "Excitement. Right before the experiment. #KitchenOceanography](https://mirjamglessmer.com/wp-content/uploads/2021/01/3.png)